Preparation method of hyaluronic acid-based bi-crosslinked hydrogel
A technology of hyaluronic acid and cross-linked hyaluronic acid, which is applied in the fields of pharmaceutical formulations, medical science, and prostheses, and can solve problems such as insufficient mechanical properties of injectable hydrogels, limited tissue engineering applications, and poor tissue adhesion. Achieve the effects of ensuring non-toxicity and cell compatibility, simple and easy-to-operate preparation process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
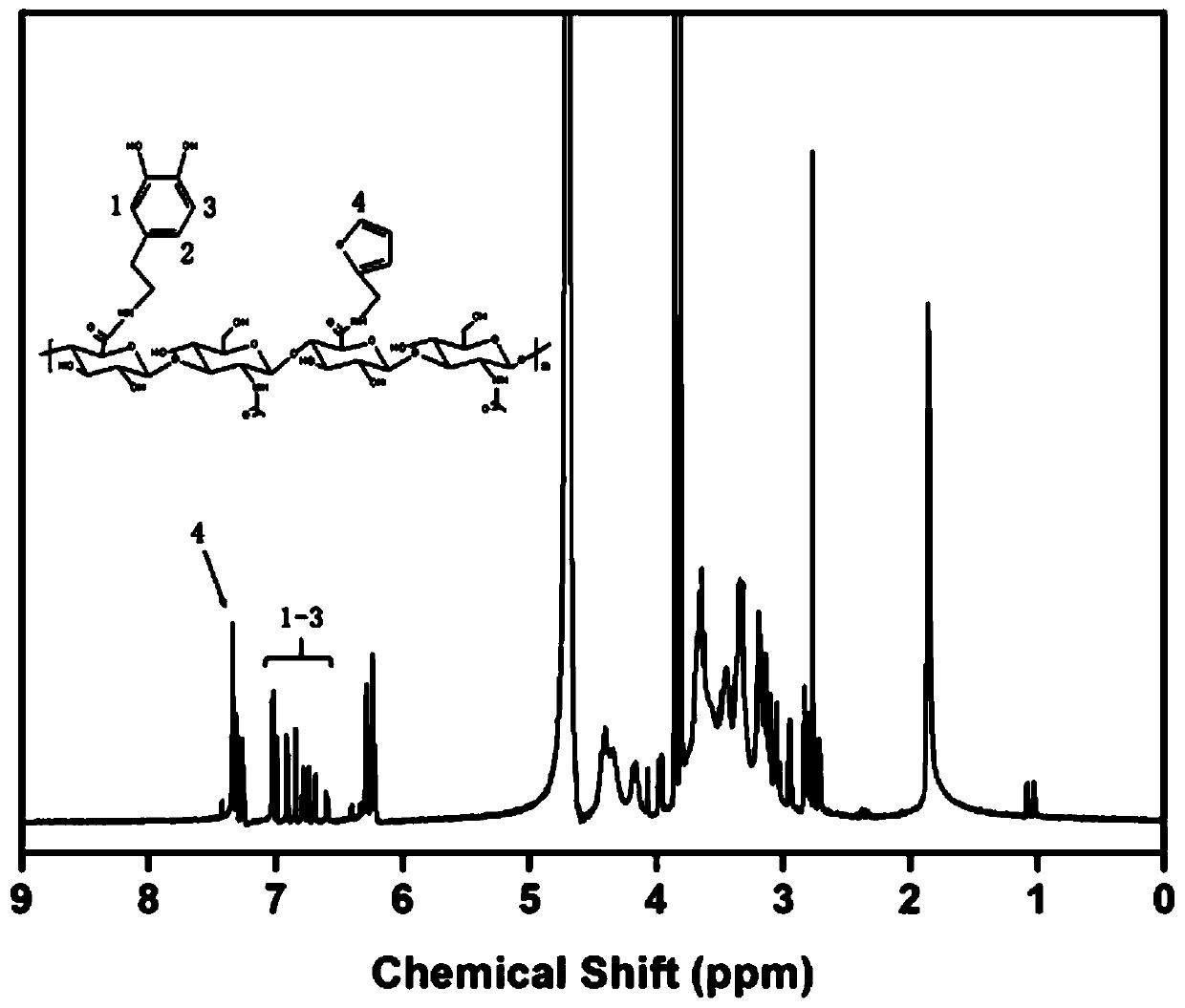
[0030] Dissolve 0.5g hyaluronic acid completely in 100mM morpholineethanesulfonic acid buffer at room temperature, add 0.7g carboxyl activator 4-(4,6-dimethoxytriazin-2-yl)-4-methanol After activating the carboxyl group with morpholine hydrochloride for 30 minutes, add 110 μL of furfurylamine, stir and react for 24 hours at room temperature in the dark, then add 0.7 g of 4-(4,6-dimethoxytriazin-2-yl)-4-methyl After activating the carboxyl group with morpholine hydrochloride for 30 minutes, add 0.7112 g of dopamine hydrochloride, stir and react for 24 hours, after the reaction, dialyze through a dialysis bag with a molecular weight cut-off of 8000-14000 for 4 days, and freeze-dry at -50°C to obtain furan and dopamine-modified hyaluronic acid .
[0031] Dissolve 0.5g hyaluronic acid completely in 100mM morpholineethanesulfonic acid buffer at room temperature, add 0.7g carboxyl activator 4-(4,6-dimethoxytriazin-2-yl)-4-methanol After activating the carboxyl group with morpholine...
Embodiment 2
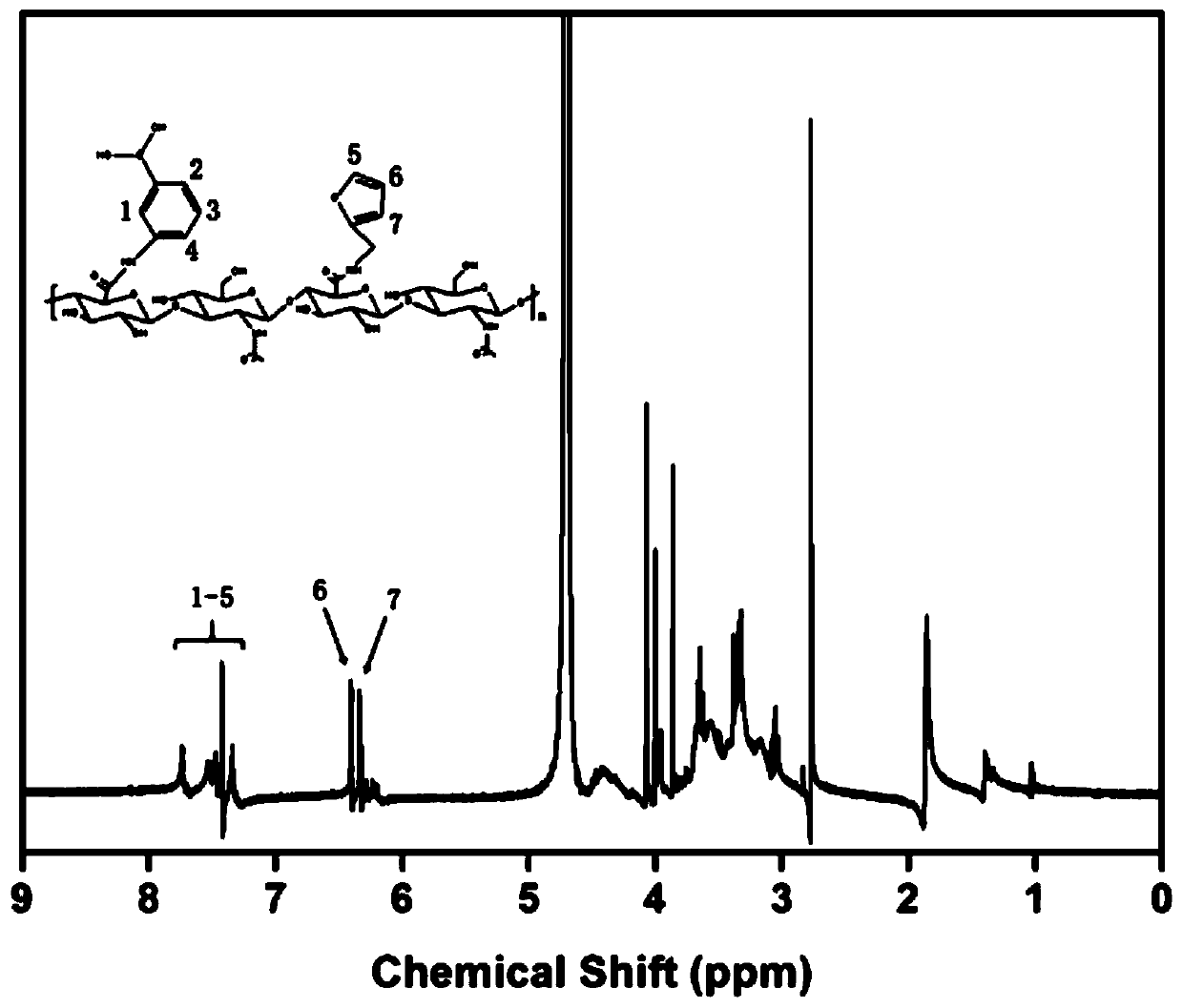
[0037] Dissolve 0.5g hyaluronic acid completely in 100mM morpholineethanesulfonic acid buffer at room temperature, add 1.4g carboxyl activator 4-(4,6-dimethoxytriazin-2-yl)-4-methanol After activating the carboxyl group with morpholine hydrochloride for 30 minutes, add 110 μL of furfurylamine, stir and react for 24 hours at room temperature in the dark, then add 1.4 g of 4-(4,6-dimethoxytriazin-2-yl)-4-methyl After activating the carboxyl group with morpholine hydrochloride for 30 minutes, add 0.7112 g of dopamine hydrochloride, stir and react for 24 hours, after the reaction, dialyze through a dialysis bag with a molecular weight cut-off of 8000-14000 for 4 days, and freeze-dry at -50°C to obtain furan and dopamine-modified hyaluronic acid .
[0038] Dissolve 0.5g hyaluronic acid completely in 100mM morpholineethanesulfonic acid buffer at room temperature, add 1.4g carboxyl activator 4-(4,6-dimethoxytriazin-2-yl)-4-methanol After activating the carboxyl group with morpholine...
Embodiment 3
[0041] Example 3 (compression performance test of double cross-linked hydrogels at different pHs)
[0042] The 2% double cross-linked hydrogel was soaked in phosphate buffer solution with pH 6, 7.4 and 9 for 24 hours to make it swell completely. A dynamic mechanical analyzer was used to test the compressive properties of hydrogels that swelled completely under different pH phosphate buffers, and the obtained compression curves were as follows: Figure 5 shown. The results indicated that the mechanical properties of the hydrogel improved with the increase of pH. This is because as the pH increases, the more phenylboronate is formed, which improves the mechanical properties of the hydrogel to a certain extent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Shear modulus | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com