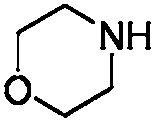
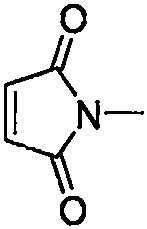
Synthesis method of 3-alkylmercapto-1-methyl-4-morpholinyl maleimide compound
A technology of methylmaleimide and maleimide, applied in the field of organic compound synthesis, can solve problems such as unstable pharmacological activity or biological activity research, achieve important social and economic significance, simple reaction conditions, The effect of high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 3-(2-ethoxyethylmercapto)-1-methyl-4-morpholinomaleimide
[0033]
[0034] At room temperature, substituted 2-ethoxyethylmercapto Buente salt (0.6mmol, 3equiv), morpholine (0.4mmol, 2equiv), N-methylmaleimide (0.2mmol, 1equiv), iodine Cuprous chloride (0.02 mmol, 0.1 equiv) and 2 mL of 1,2-dichloroethane were added to the reaction tube, then filled with oxygen, and replaced three times, and stirred at a reaction temperature of 100° C. for 24 h. The reaction mixture was cooled, then diluted with ethyl acetate, filtered to a heart bottle, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether = 9: 1), and the product was a yellow solid. The melting point is 51-52° C., the yield is 95%, and the product weight is 57 mg.
[0035] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0036] 1 H NMR (500MHz, CDCl 3 ): δ4.20(t, J=4.70Hz, 4H), 3.79(t, J=4.7...
Embodiment 2
[0042] Synthesis of 3-decylmercapto-1-methyl-4-morpholinomaleimide
[0043]
[0044] At room temperature, substituted decylmercapto Buente salt (0.6mmol, 3equiv), morpholine (0.4mmol, 2equiv), N-methylmaleimide (0.2mmol, 1equiv), cuprous iodide (0.02 mmol, 0.1 equiv) and 2 mL of 1,2-dichloroethane were added to the reaction tube, then filled with oxygen, and replaced three times, and stirred at a reaction temperature of 100°C for 24h. The reaction mixture was cooled, then diluted with ethyl acetate, filtered to a heart bottle, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether = 9: 1), and the product was a yellow solid. The melting point is 49-50° C., the yield is 92%, and the product weight is 68 mg.
[0045] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0046] 1 H NMR (500MHz, CDCl 3 ): δ4.18(t, J=4.70Hz, 4H), 3.79(t, J=4.70Hz, 4H), 2.99(s, 3H), 2.70(t, J...
Embodiment 3
[0052] Synthesis of 3-butylmercapto-1-methyl-4-morpholinylmaleimide
[0053]
[0054] At room temperature, substituted butylmercapto Buendt's salt (0.6mmol, 3equiv), morpholine (0.4mmol, 2equiv), N-methylmaleimide (0.2mmol, 1equiv), cuprous iodide (0.02 mmol, 0.1 equiv) and 2 mL of 1,2-dichloroethane were added to the reaction tube, then filled with oxygen, and replaced three times, and stirred at a reaction temperature of 100°C for 24h. The reaction mixture was cooled, then diluted with ethyl acetate, filtered to a heart bottle, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether = 9: 1), the product was a yellow liquid, The yield is 88%, and the product weight is 50 mg.
[0055] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0056] 1 H NMR (500MHz, CDCl 3 ): δ4.19(t, J=4.70Hz, 4H), 3.79(t, J=4.70Hz, 4H), 2.99(s, 3H), 2.71(t, J=7.40Hz, 2H), 1.56-1.52 (m, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com