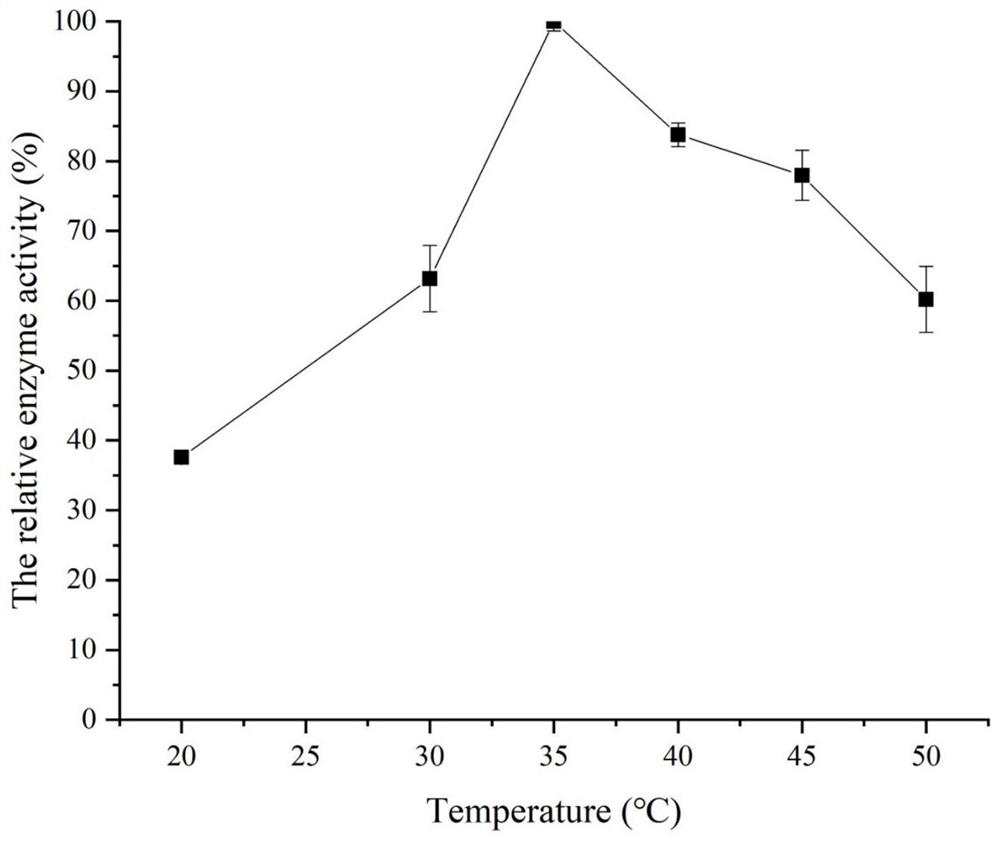
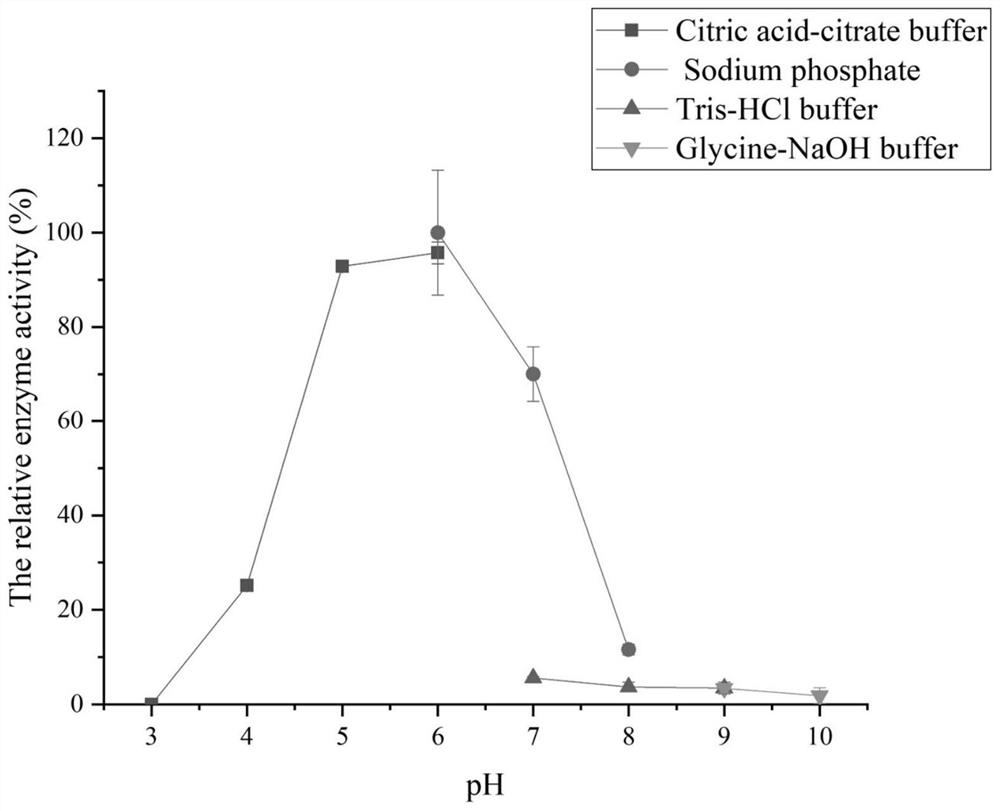
Laminarinase ouc-l1 and its coding gene and application
A technology of OUC-L1 and laminarinase, applied in laminarinase OUC-L1 and its encoding gene and application field, can solve the problems of biological activity destruction of laminarin, difficult to control the degree of degradation, difficult to separate and purify, etc., and achieve good biological performance. The effect of catalytic efficiency, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
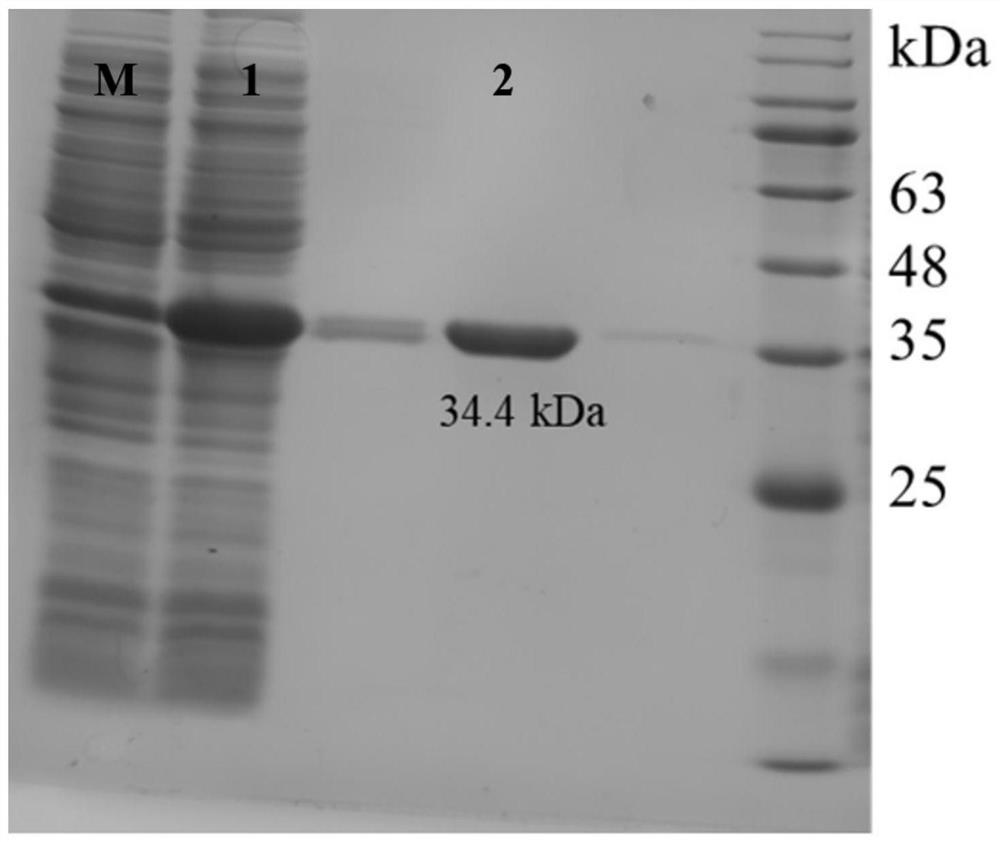
[0024] Example 1 Cloning of Laminarinase OUC-L1
[0025] The enzyme-producing gene of laminarinase OUC-L1 of the present invention is based on human intestinal bacterium A.muciniphila (gifted by Mr. Pang Xiaoyang, Institute of Agricultural Products Processing, Chinese Academy of Agricultural Sciences) genome (although the genome sequence of this bacterium has been disclosed, but about this The laminarinase OUC-L1 gene involved in the invention has not been isolated or amplified separately and its biological activity has been characterized; this invention is the first time that OUC-L1 has been isolated from the genome of human intestinal microorganism Akkermansia muciniphila gene) as a template, obtained through PCR specific amplification:
[0026] Download the human intestinal bacterium A.muciniphila genome sequence (Accession number: GCA_000020225.1) from NCBI, and use the BioEdit software to convert the characterized laminarin enzyme gene sequence such as LamC (Accession num...
Embodiment 2
[0034] Embodiment 2 Containing the expression vector construction of laminarinase gene
[0035] The gene fragment and the pET28a(+) cloning vector were connected by seamless cloning technology, and the connection product was transferred into E.coli DH5α competent cells, and spread on the LB medium solid plate containing 50 μg / mL kanamycin. After culturing in a 37°C incubator for 12-16 hours, pick a single clone into LB liquid medium containing 50 μg / mL kanamycin, culture overnight on a shaker at 37°C at a speed of 220 rpm, and perform sequencing after PCR positive verification. The bacterial liquid verified by sequencing was placed in a glycerol tube for storage at -20°C, and the expression vector was named pET28a-OUC-L1.
Embodiment 3
[0036] Embodiment 3 Containing the recombinant plasmid of laminarinase gene and the construction of engineering bacteria
[0037] The recombinant pET28a-OUC-L1 plasmid was obtained by extracting the bacterial liquid with correct sequencing, which was transformed into the host E.coli BL21 competent cells, and the constructed engineering bacteria were grown on the plate containing 50 μg / mL kanamycin resistance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com