Compositions and methods of use for treating aberrant inflammation in peri-ocular secretory glands or at the ocular surface
A composition and ocular surface technology, applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as delayed healing, low corneal permeability, and poor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
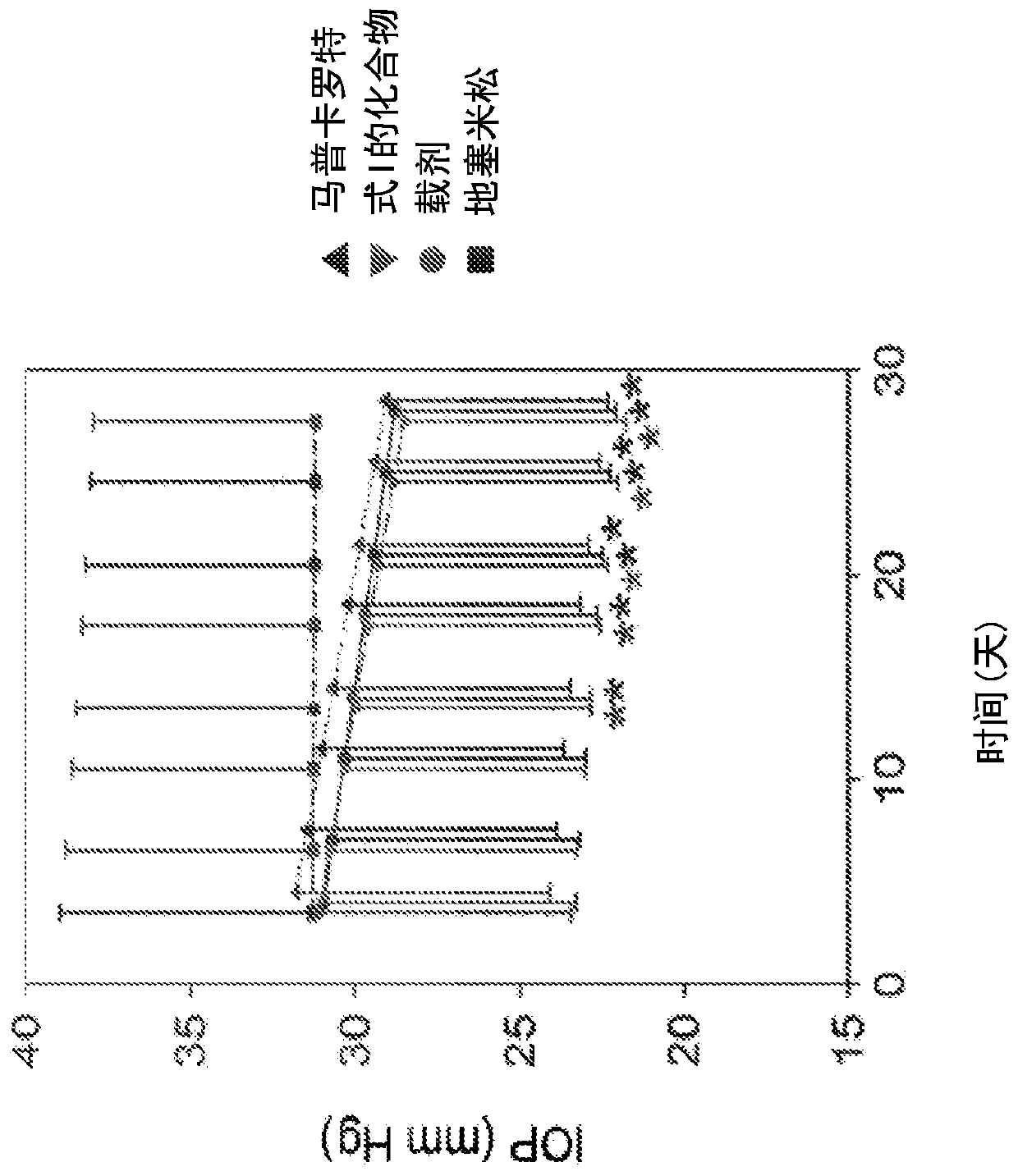
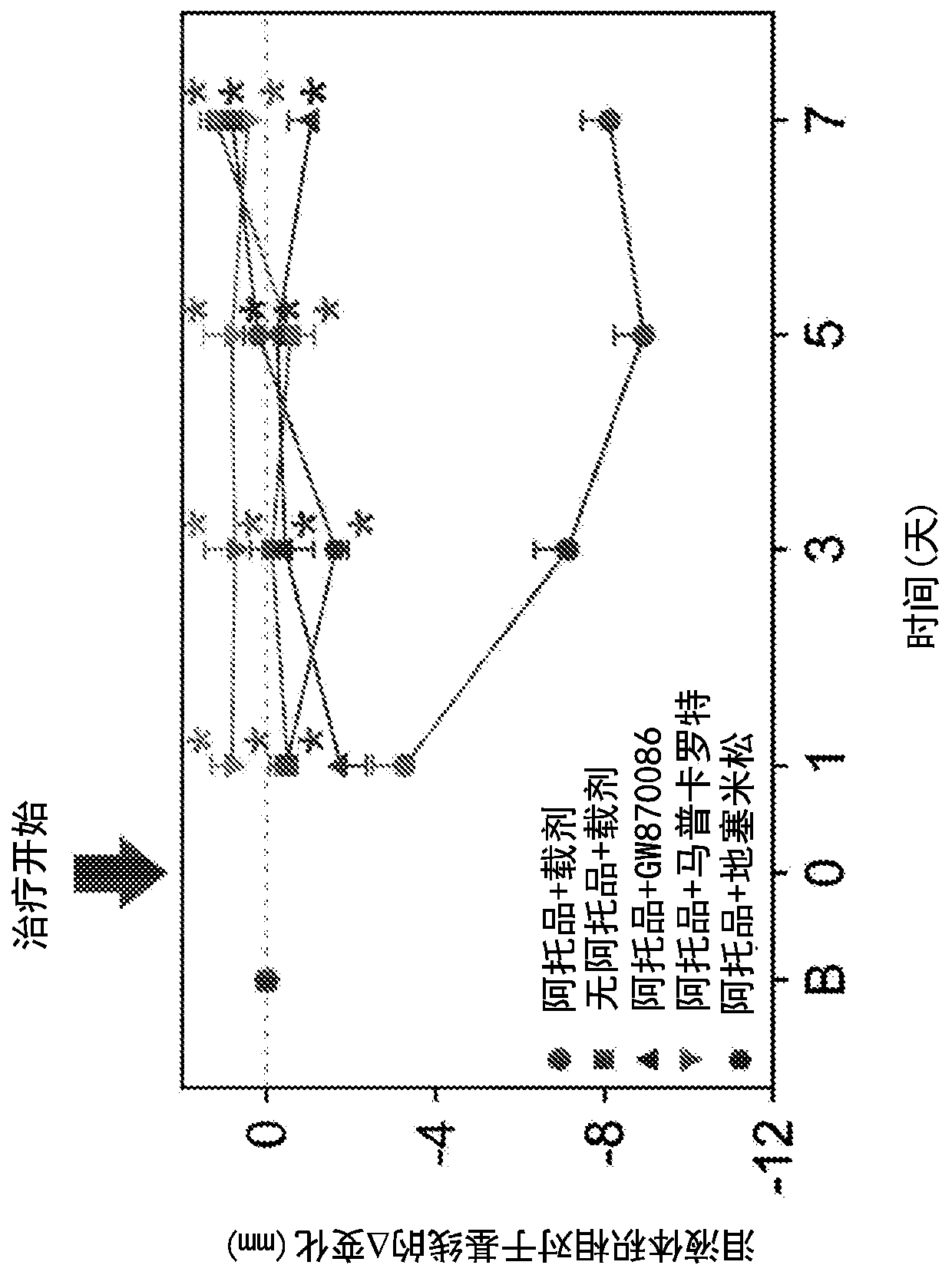
[0146] Example 1 : Effects of treatment with compounds of formula I on intraocular pressure (IOP) and tear formation
[0147] Compounds of formula I are fluorinated glucocorticoids with anti-inflammatory activity (see US Pat. No. 7,288,536). Compounds of formula I exhibit near full agonism in various in vitro assays of human glucocorticoid receptor-mediated counter-inhibitory activity, and in many assays of glucocorticoid receptor-mediated transactivation Partial response. In vivo, compounds of formula I show potent anti-inflammatory activity following intratracheal administration in mouse and rat models of pulmonary inflammation and local delayed-type hypersensitivity ear inflammation in mice , tyrosine aminotransferase-induced and chronic house dust mite models exhibit similar activity to fluticasone propionate (FP). Interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNFα) stimulate the pro-inflammatory cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8) in multip...
Embodiment 2
[0153] Example 2: Determination of properties of periocular cream formulations
[0154] In this study, the following active pharmaceutical ingredients (APIs) were mixed with a cream vehicle to prepare a cream containing 2 wt% API and allowed to stand at room temperature for 24 hours prior to testing. Cyclosporine A (Sample A), Loteprednol Ecarb (Sample B), Dexamethasone (Sample C), Ketorolac Trimethylamine (Sample D), Gatifloxacin, USP (Sample E), Gentamicin sulfate (sample F), prednisolone (sample G), flurbiprofen (sample H), azithromycin dihydrate (sample J), triamcinolone acetonide (sample K), and doxycycline hydrochloride (Sample L). The carrier cream contains 48% by weight of white petrolatum, 8% by weight of mineral oil, 8% by weight of propylene glycol, 6.6% by weight of ST-Cyclomethicone-5NF, 3.3% by weight of Emulsifier-10, 2% by weight of ST-Elastomer-10, 0.08% by weight methylparaben, 0.06% by weight disodium hydrogen phosphate anhydrous, 0.0546% by weight anhyd...
Embodiment 3
[0163] Example 3: Skin Penetration of Eye Cream
[0164] An in vitro skin penetration model of human cadaver skin mounted in a Franz-type diffusion cell (FDC) was chosen to determine the skin permeability of a 2% cream of the compound of formula I applied to the epidermal surface. Transdermal flux into the receiving fluid was measured within 46 hours after application of the formulation. At the end of the 46 hour incubation period, the skin was tape stripped and thermally separated based on established methods, and the concentration of the compound of formula I was measured in the remaining epidermis and dermis. The applied formulation contained: 2% by weight of compound of formula I, 46% by weight of white petrolatum, 8% by weight of mineral oil, 8% by weight of propylene glycol, 6.6% by weight of ST-cyclomethicone-5NF, 3.3% by weight of Emulsifier-10, 2 wt% ST-Elastomer-10, 0.08 wt% methylparaben, 0.06 wt% disodium hydrogen phosphate anhydrous, 0.05 wt% anhydrous citric aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com