Ultraviolet-excited fluorescent dye and application thereof
A technology for exciting fluorescence and ultraviolet light, which is applied in the field of fluorescent dyes, can solve the problems of reduced fluorescence quantum yield, reduced sensitivity, and increased dye dosage, and achieves the effects of stable solubility, increased solubility, and reduced negative charge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
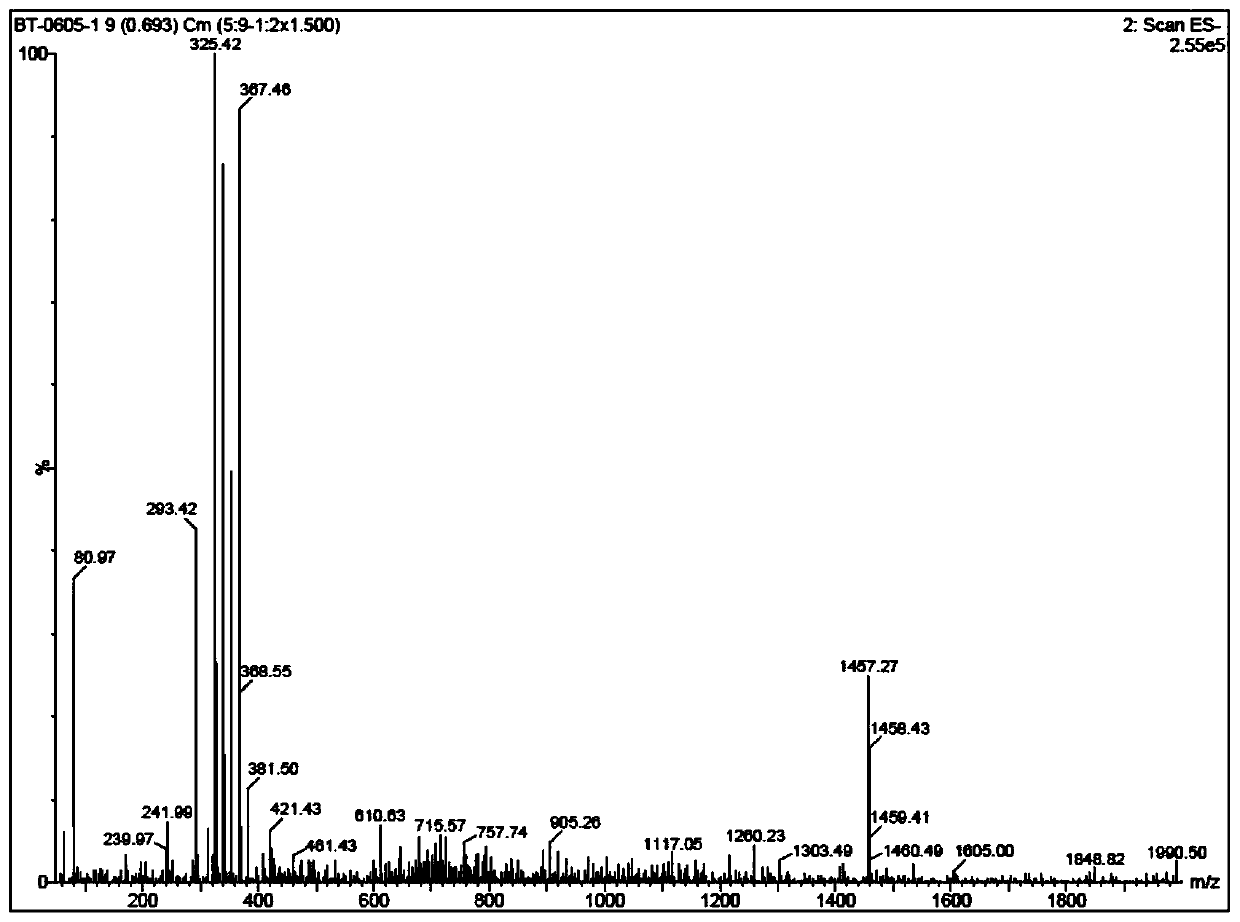
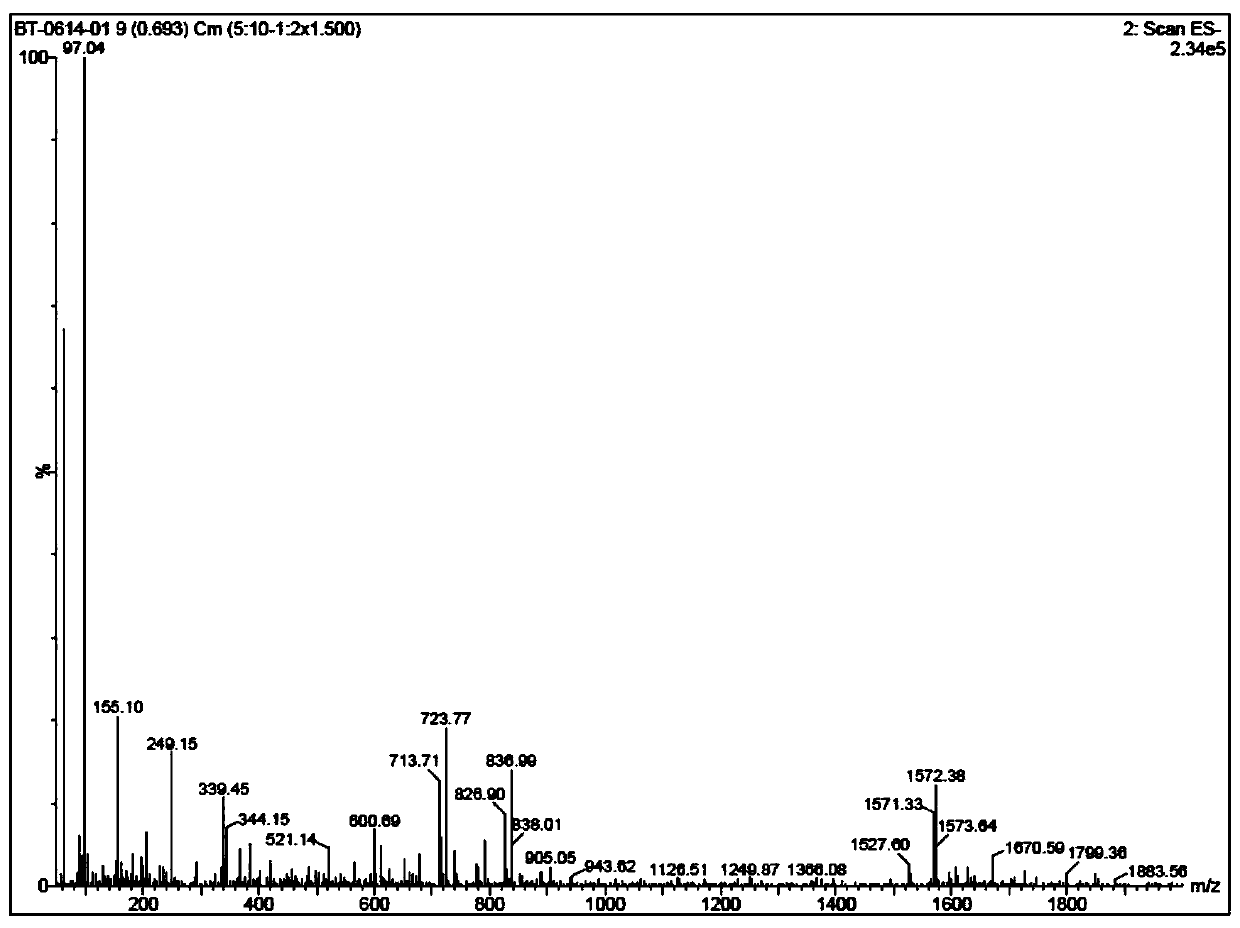
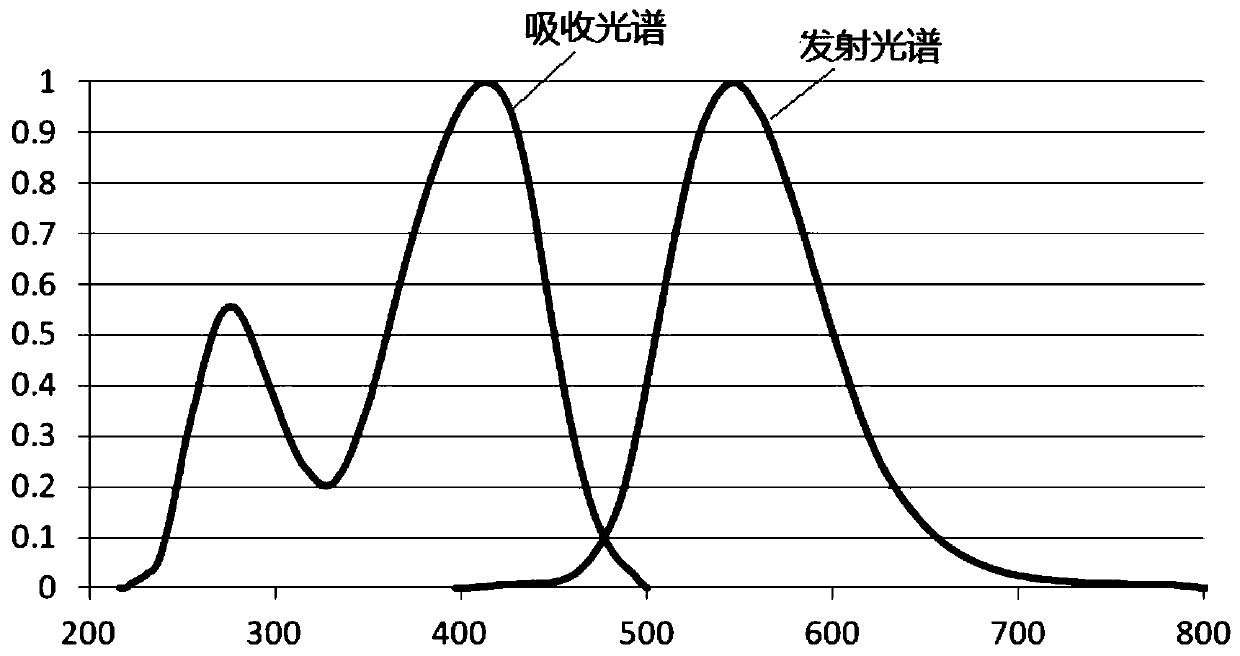
Image
Examples
preparation example Construction
[0098] Preparation of "dye-conjugates"
[0099] A method for coupling an ultraviolet light-excited fluorescent dye of the present invention to a carrier molecule or a solid support, comprising the steps of:
[0100] (1) contacting the carrier molecule or solid support with the dye to form a combined sample;
[0101] (2) incubating the combined sample for a certain period of time to form a covalent bond between the compound of the general formula I and the carrier molecule or solid support to obtain a mixture of dye-conjugates;
[0102] (3) Separating the compound not connected to the carrier molecule or the solid support from the compound connected to the carrier molecule or the solid support in the dye-conjugate mixture to obtain the dye-conjugate.
[0103] Conjugates of dyes and carrier molecules or solid supports, for example: drugs, polypeptides, toxins, nucleic acids, phospholipids and other organic substances prepared by organic synthesis methods and reactive dyes of th...
Embodiment 1
[0123] Embodiment 1: the preparation of compound 5:
[0124] (1) Synthesis of compound 1:
[0125]
[0126] According to the synthesis method of compound 6 in US2012004397, compound 1 was prepared.
[0127] Compound 1 was subjected to nuclear magnetic resonance (NMR) characterization, and the characterization results were as follows:
[0128] Compound 1: 1 H NMR: 1.1(t,3H),1.3-3(m,8H),4-5(m,4H),7.03(d,2H),7.1(S,1H),7.66(d,2H),7.9 -8.8(m,4H)
[0129] (2) Synthesis of compound 2:
[0130]
[0131] 1g of compound 1 (2.63mmol) was suspended in 10ml of concentrated hydrochloric acid, and reacted overnight at 65°C. The reaction was followed by TLC. After the reaction, the hydrochloric acid and water were removed by rotary evaporation, and it could be used in the next reaction without drying. A small amount was subjected to column chromatography and the resulting solid product, compound 2, was sucked dry.
[0132] Compound 2 was characterized by NMR, and the characterizat...
Embodiment 2
[0150] Preparation of compound 6A:
[0151]
[0152] 1 g of compound 5 (0.64 mmol) was dissolved in 20 ml of anhydrous DMA, 0.16 g of DSC and 6.2 mg of DMAP were added to the reaction solution, and the suspension was stirred at room temperature for 2 days. After the reaction was over, the solvent was concentrated to 3ml under reduced pressure, 300ml ethyl acetate was added and stirred for 0.5h, the solid was collected, filtered and washed with ethyl acetate to obtain compound 6.
[0153] Compound 6A was subjected to NMR characterization, and the characterization results were as follows:
[0154] Compound 6A: 1 H NMR: 1.3-2.5(m,20H),3-5(m,99H),7.5(d,1H),7.1(S,1H),8.1(d,1H),8.3(s,1H),7.9 -8.8(m,4H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com