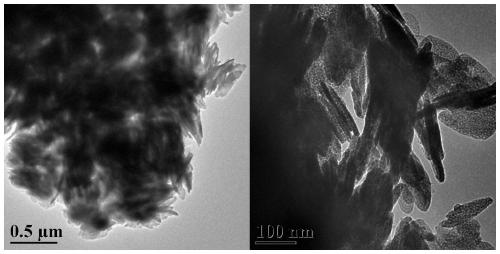
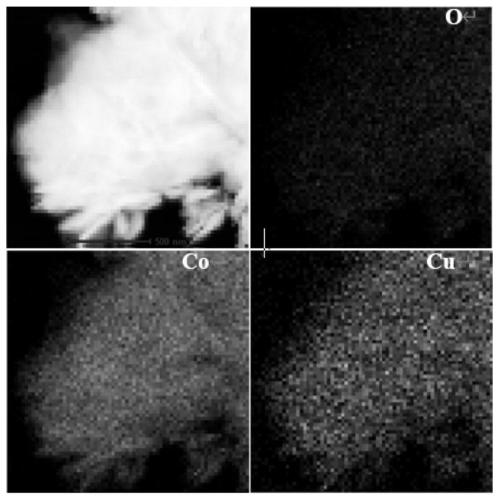
Preparation method of flaky cuprous oxide/cobaltous oxide nano composite material and application of flaky cuprous oxide/cobaltous oxide nano composite material in catalyzing hydrolysis of ammonia borane to produce hydrogen
A technology of cobaltous oxide nanometer and flaky cuprous oxide, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, catalyst activation/preparation, chemical instruments and methods, etc., and can solve the problem of high reaction temperature and hydrothermal reaction Long time, potential safety hazards and other problems, to achieve the effect of simple operation, easy industrial production, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 0.249gCuSO 4 ·5H 2 O and 0.843gCoSO 4 ·7H 2 O was dissolved in 20mL of ultrapure water, magnetically stirred to dissolve to obtain solution A, and 2.258g of potassium sodium tartrate and 0.545g of sodium dodecylsulfonate were dissolved in 20mL of ultrapure water, magnetically stirred to dissolve to obtain solution B. The B solution was slowly added dropwise to A to obtain the C solution, and the stirring was continued for 30 min. Weigh 4g of NaOH and dissolve it in 40mL of ultrapure water to obtain D solution, slowly drop D solution into C solution, continue to stir for 30min, then transfer to the reaction kettle, tighten and react under hydrothermal reaction at 160°C for 4h. After the reaction, cool to room temperature, filter and wash, collect the product, wash 2-3 times with water, wash 2-3 times with ethanol, dry in a vacuum oven at 60°C, and calcine at 250°C for 4 hours to obtain the target product sheet Cu-like 2 O-CoO nanocomposite catalysts.
Embodiment 2
[0038] Weigh 0.249gCuSO 4 ·5H 2 O and 0.843gCoSO 4 ·7H 2O was dissolved in 20mL of ultrapure water, magnetically stirred to dissolve to obtain solution A, and 1.553g of sodium tartrate and 0.545g of sodium dodecylsulfonate were dissolved in 20mL of ultrapure water, magnetically stirred to dissolve to obtain solution of B. The B solution was slowly added dropwise to A to obtain the C solution, and the stirring was continued for 30 min. Weigh 4g of NaOH and dissolve it in 40mL of ultrapure water to obtain D solution, slowly drop D solution into C solution, continue to stir for 30min, then transfer to the reaction kettle, tighten and react under hydrothermal reaction at 160°C for 4h. After the reaction, cool to room temperature, filter and wash, collect the product, wash 2-3 times with water, wash 2-3 times with ethanol, dry in a vacuum oven at 60°C, and calcinate at 250°C for 4 hours to obtain the target product sheet Cu-like 2 O-CoO nanocomposite catalysts.
Embodiment 3
[0040] Weigh 0.249gCuSO 4 ·5H 2 O and 0.843gCoSO 4 ·7H 2 O was dissolved in 20mL of ultrapure water, stirred and dissolved by magnetic force to obtain a solution of solution A, and 1.2g of tartaric acid and 0.545g of sodium dodecylsulfonate were dissolved in 20mL of ultrapure water, stirred and dissolved by magnetic force to obtain a solution of solution B. The B solution was slowly added dropwise to A to obtain the C solution, and the stirring was continued for 30 min. Weigh 4g of NaOH and dissolve it in 40mL of ultrapure water to obtain D solution, slowly drop D solution into C solution, continue to stir for 30min, then transfer to the reaction kettle, tighten and react under hydrothermal reaction at 160°C for 4h. After the reaction, cool to room temperature, filter and wash, collect the product, wash 2-3 times with water, wash 2-3 times with ethanol, dry in a vacuum oven at 60°C, and calcinate at 250°C for 4 hours to obtain the target product sheet Cu-like 2 O-CoO nano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com