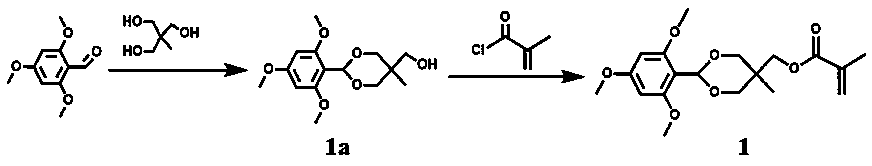
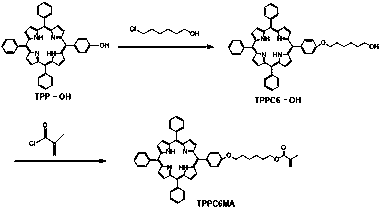
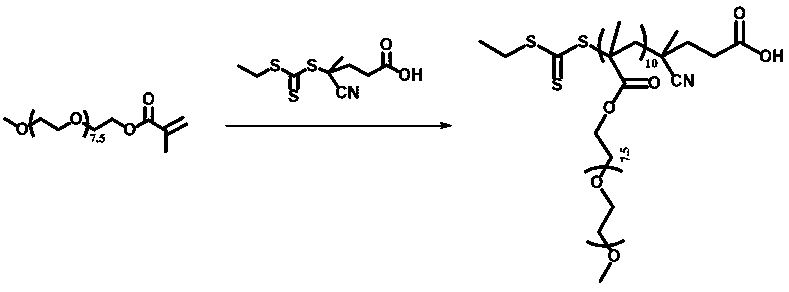
Porphyrin-containing acid-responsive photodynamic therapy polymer nano-drug
A technology of nano-medicine and acetal polymer, which is applied in the field of biomedicine, can solve problems such as the reduction of the effect of photodynamic therapy, and achieve the effects of weakening the stacking quenching effect, improving efficiency, and improving utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of amphiphilic block copolymer POEGMA-b-[PTPPC6MA-co-PTTMA](PM):
[0050] Block copolymers were prepared by subsequent raft polymerization of TPPC6MA and TTMA using POEGMA as the macromolecular RAFT agent. Add POEGMA (200 mg) TPPC6MA (150 mg), TTMA (95 mg), AIBN (1.82 mg) and 3ml THF to a dry polymerization tube equipped with a magnetic stir bar as follows. The polymerization tube was then sealed under vacuum by going through at least three freeze-thaw cycles while evacuating nitrogen. The degassed solution was immersed in an oil bath at 70° C. and stirred at a constant temperature for 48 hours, and then the polymerization tube was treated with liquid nitrogen to freeze the solution. The mixture was then precipitated three times in excess diethyl ether and dried in a vacuum oven at room temperature. Final product (181 mg, 35%).
Embodiment 2
[0059]Self-Assembly of Amphiphilic Block Copolymers:
[0060] Dissolve 10 mg of the copolymer in 10 ml of tetrahydrofuran to prepare an organic concentrated solution of 1 mg / ml. Then, the copolymer solution (1 mg) was slowly dropped into 4.0 ml phosphate buffered saline (PBS, 10 mm, pH=7.4) under stirring with a magnetic stirrer, and this process lasted for 1 h. After further stirring for 1 h, micelles formed. The resulting solution was stirred overnight and THF was removed by dialysis (MWCO 3.5 kDa) against PBS buffer (pH=7.4) for 3 days.
[0061] The invention obtains three kinds of porphyrin-containing polymer nano drug micelles, two of which have innovative acetal structures. The ultraviolet absorption test was carried out, and it was found that the position of the ultraviolet absorption peak of the substance changed only slightly before and after assembly, indicating that the structure of the chemical substance did not change significantly before and after assembly, and...
Embodiment 3
[0063] Cell uptake test:
[0064] Cellular drug uptake was detected by confocal laser scanning microscopy (CLSM). Firstly, cells were plated in culture dishes, 10,000 cells per dish, and 2 ml medium was added for culture. After 24 hours, the culture solution was taken out, and 2 ml of the prepared medium containing the drug (porphyrin) concentrate at a concentration of 25ug / ml was added to a six-well plate, and cultured for 4 hours and 24 hours, respectively. Afterwards, remove the medium, wash the cells with PBS 3 times, then add 4% paraformaldehyde solution to the culture dish, and wait for 20 minutes. Cells were then rinsed again with PBS to remove excess paraformaldehyde, DAPI was added to the dish for nuclear staining, and waited 3 min. Finally, the cells were washed with PBS to remove excess DAPI, and the cells were imaged with a laser confocal microscope. The results showed that the three polymer drug micelles could be well absorbed by the cells, and the drug concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com