Preparation method of 1-phenyl adamantane and obtained 1-phenyl adamantane
A technology of phenyladamantane and adamantane, which is applied in the field of 1-phenyladamantane, can solve the problems of complex reaction and low yield, and achieve the effect of high reaction yield, low reaction temperature and green production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
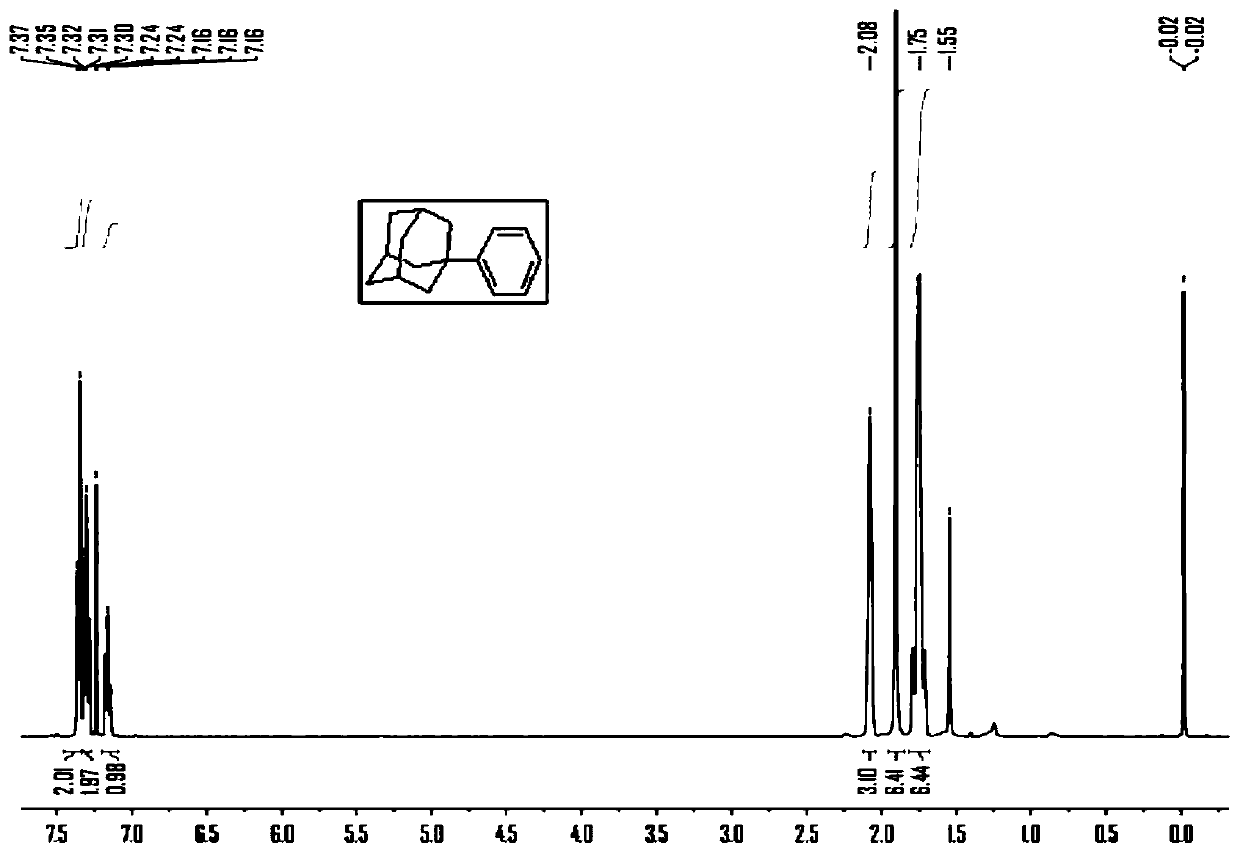
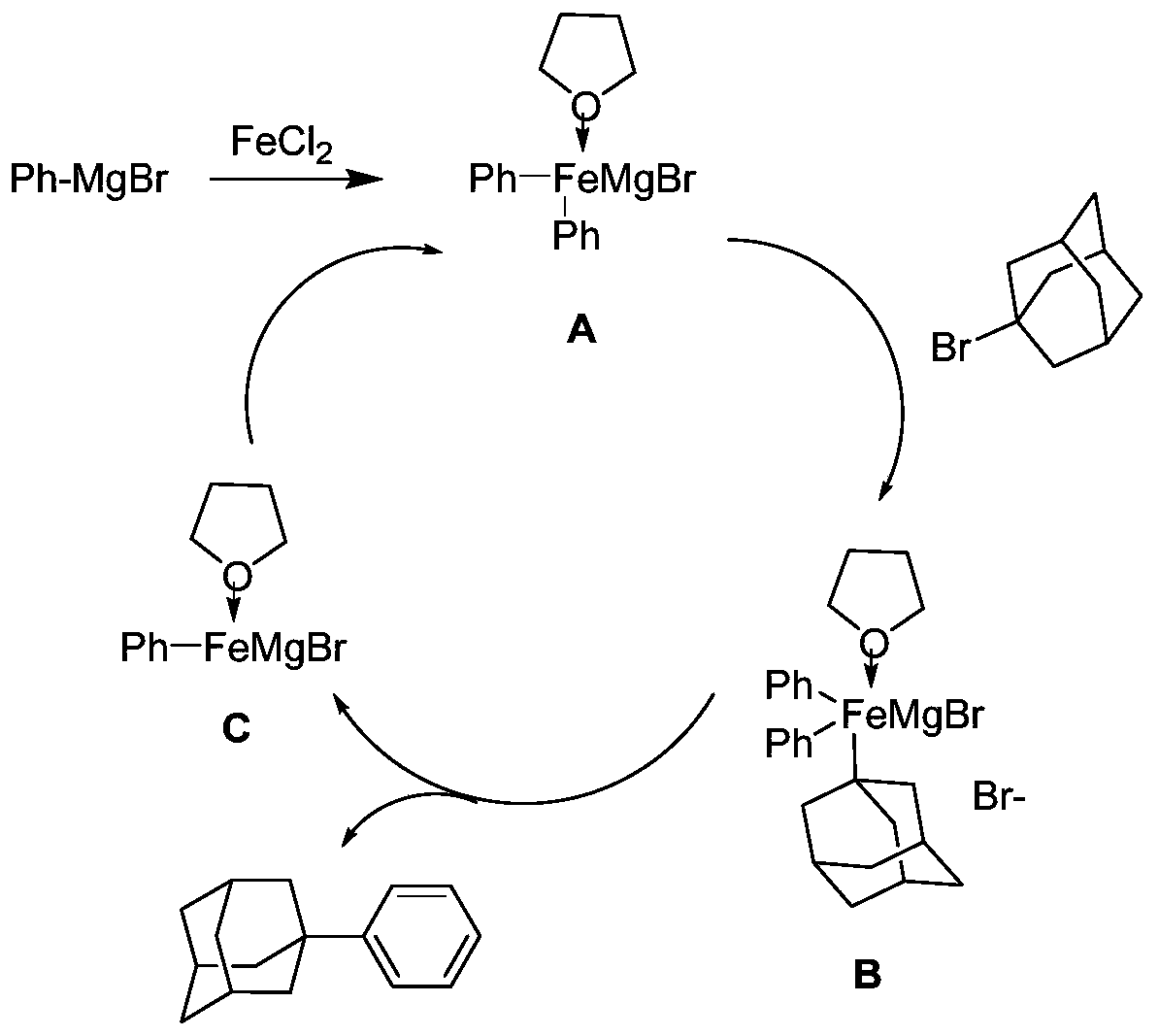
[0043]Under the protection of nitrogen, add magnesium chip (2.64g, 0.11mol), 0.05g iodine, 100ml of anhydrous tetrahydrofuran to the 500ml four-neck flask equipped with mechanical stirrer, reflux condenser, constant pressure dropping funnel, thermometer, and heat to At 60°C, bromobenzene (15.7 g, 0.1 mol) was added dropwise to keep the solvent slightly boiling to obtain 0.1 mol of bromobenzene Grignard reagent. Then continue to drop 150ml tetrahydrofuran solution of 1-bromoadamantane (32.25g, 0.15mol), add anhydrous ferrous chloride (0.635g, 0.005mol), be incubated at 60 degree of reaction 24 hours, as image 3 Shown is ferrous chloride as the reaction mechanism schematic diagram of catalyzer, TLC monitors that reaction finishes, is cooled to room temperature, adds saturated ammonium chloride aqueous solution to quench reaction, extracts organic phase with ethyl acetate, and saturated sodium chloride aqueous solution washes organic phase, Dry over anhydrous magnesium sulfate, ...
Embodiment 2
[0046] Under the protection of nitrogen, magnesium chips (2.64g, 0.11mol), 0.05g iodine, and 100ml of anhydrous ether were added to a 500ml four-necked flask equipped with a mechanical stirrer, a reflux condenser, a constant pressure dropping funnel, and a thermometer, and heated to At 30°C, bromobenzene (15.7 g, 0.1 mol) was added dropwise to keep the solvent slightly boiling to obtain 0.1 mol of bromobenzene Grignard reagent. Then continue to dropwise add the 150ml diethyl ether solution of 1-bromoadamantane (32.25g, 0.15mol), add anhydrous ferrous chloride (0.635g, 0.005mol), be incubated at 35 degree of reaction 24 hours, as image 3 Shown is ferrous chloride as the reaction mechanism schematic diagram of catalyzer, TLC monitors that reaction finishes, is cooled to room temperature, adds saturated ammonium chloride aqueous solution to quench reaction, extracts organic phase with ethyl acetate, and saturated sodium chloride aqueous solution washes organic phase, Dry over an...
Embodiment 3
[0048] Under the protection of nitrogen, add magnesium chip (2.64g, 0.11mol), 0.05g iodine, 100ml of anhydrous tetrahydrofuran to the 500ml four-neck flask equipped with mechanical stirrer, reflux condenser, constant pressure dropping funnel, thermometer, and heat to At 60°C, bromobenzene (15.7 g, 0.1 mol) was added dropwise to keep the solvent slightly boiling to obtain 0.1 mol of bromobenzene Grignard reagent. Then continue to drop 150ml tetrahydrofuran solution of 1-bromoadamantane (32.25g, 0.15mol), add cuprous chloride (0.99g, 0.01mol), and keep warm at 60 degrees for 24 hours, as Figure 4 Shown is cuprous chloride as the reaction mechanism schematic diagram of catalyzer, TLC monitoring reaction finishes, is cooled to room temperature, adds saturated ammonium chloride aqueous solution to quench the reaction, extracts the organic phase with ethyl acetate, and saturated sodium chloride aqueous solution washes the organic phase, Dry over anhydrous magnesium sulfate, filter,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com