Injectable hyaluronic acid-based hydrogel and preparation method thereof
A technology of hyaluronic acid and sodium hyaluronate, which is applied in the field of injectable hyaluronic acid-based hydrogel and its preparation, can solve the problems of harsh synthesis conditions, complicated synthesis steps, etc., and achieves simple preparation method, mild preparation conditions, The effect of high adjustable space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of described linking agent comprises the following steps:
[0052] A) reacting 1,3-dimethoxy-1,3-dihydroisobenzofuran-5-carboxylic acid succinimide ester with polyethylene glycol whose end group is an amino group to obtain a reaction product;
[0053] B) Deprotecting the reaction product to obtain a crosslinking agent.
[0054] Wherein, the reaction process is as follows:
[0055]
[0056] In the present invention, 1,3-dimethoxy-1,3-dihydroisobenzofuran-5-carboxylic acid succinimide ester and polyethylene glycol whose end group is amino are dissolved in an organic solvent, Reaction in the presence of the agent, precipitation, to obtain the reaction product.
[0057] The molar equivalent of the 1,3-dimethoxy-1,3-dihydroisobenzofuran-5-carboxylic acid succinimide ester is 1.2 to 5 molar equivalents of the amino group in polyethylene glycol whose end group is an amino group. times, preferably 2 times; the organic solvent is preferably anhydrous...
Embodiment 1
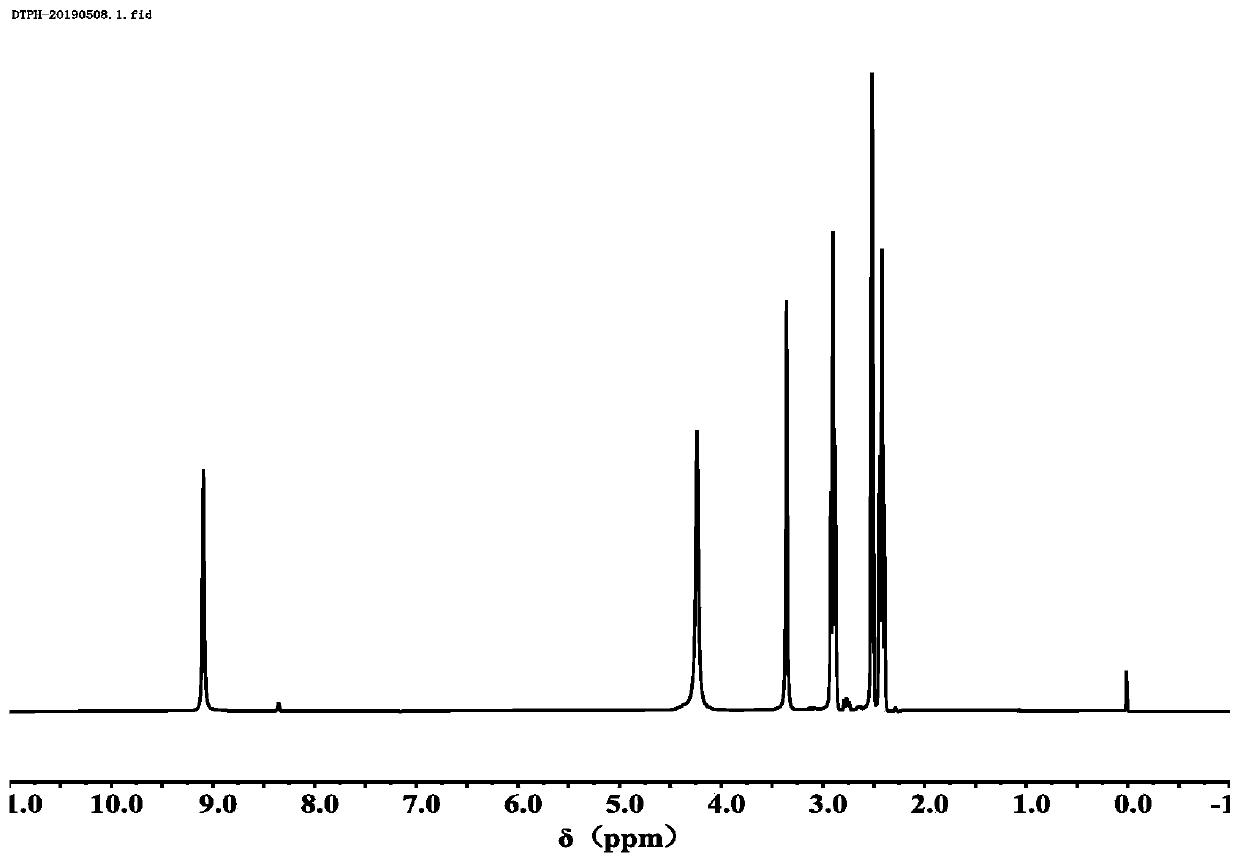
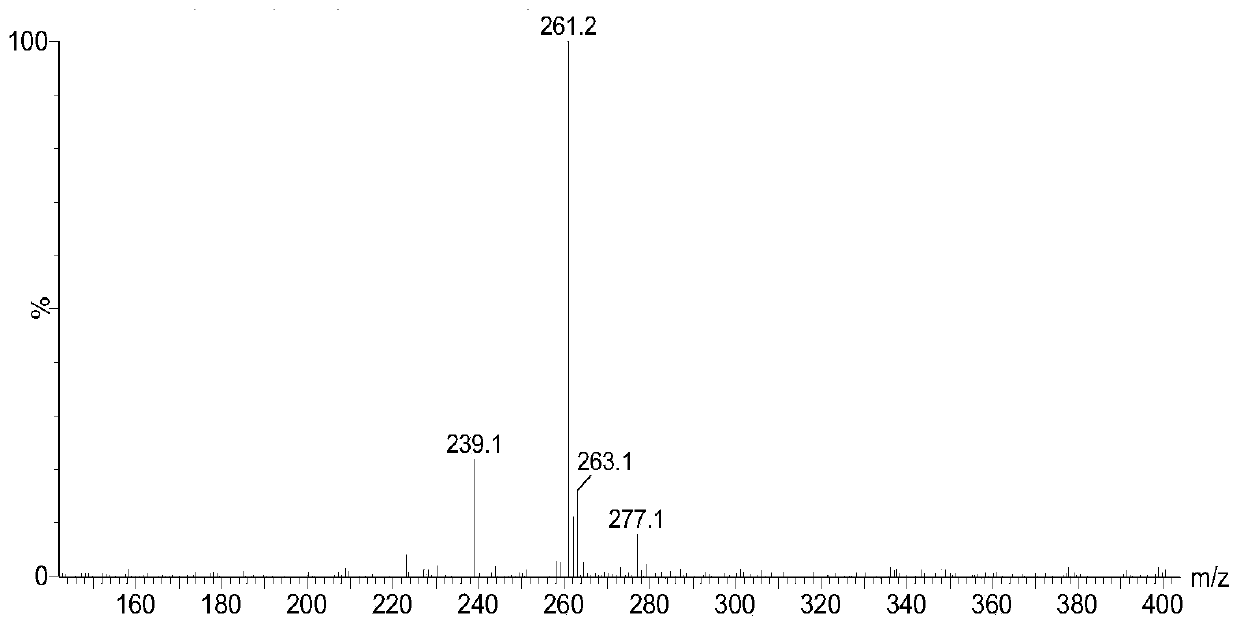
[0120] in N 2 Under atmosphere, add 12.0g of hydrazine hydrate and 20mL of absolute ethanol solution to the round bottom flask, adjust the temperature of the oil bath to 90°C, add 10.0g of dimethyl 3,3'-dithiodipropionate, stir and reflux After 5 hours, the obtained product was recrystallized three times from a mixed solution of ethanol: water = 5% (V / V) to prepare 3,3'-dithiobis(hydrazine propionate). see figure 1 and figure 2 , figure 1 For the preparation of 3,3'-dithiobis(hydrazine propionate) prepared in Example 1 of the present invention; figure 2 The mass spectrum of 3,3'-dithiobis(hydrazine propionate) prepared for Example 1 of the present invention.
Embodiment 2
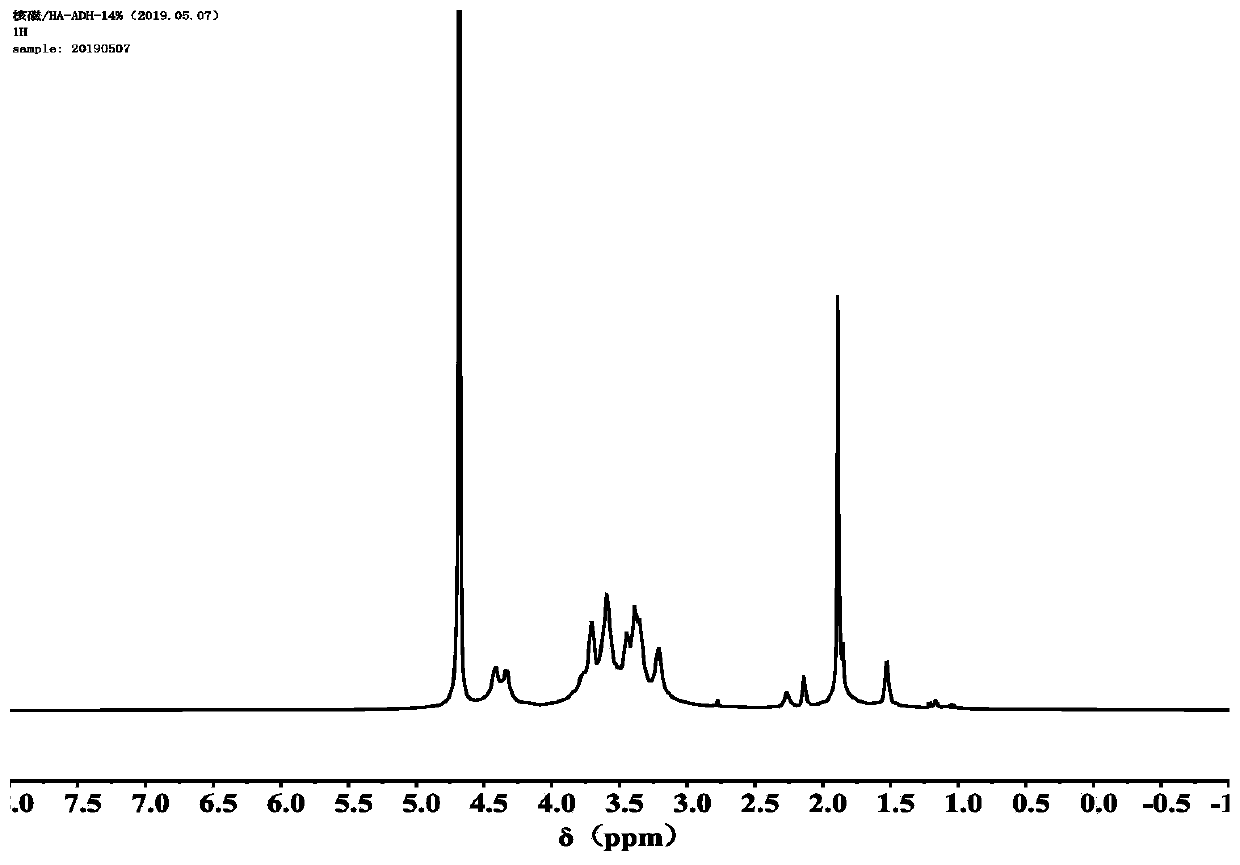
[0122] Weigh 1.2g of sodium hyaluronate and add it into 200mL of ultrapure water and stir until it is completely dissolved, then add 5.2g of adipic acid dihydrazide, 0.4g of 1-hydroxybenzotriazole, 58mg of 1-(3-dimethylamino Propyl)-3-ethylcarbodiimide hydrochloride, stir to make it completely dissolved, then use 0.01M dilute hydrochloric acid solution to adjust the pH of the system to 4.7, stir and react at 25°C for 72 hours, and use a 7000Da dialysis bag to obtain the product After dialysis for 72 hours, the product hyaluronic acid-graft-adipate dihydrazide was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com