Method for inducing differentiation of adipose derived stem cells into chondrocyte
An adipose stem cell and induction differentiation technology, which is applied in the field of adipose stem cells to induce differentiation into chondrocytes, can solve the problems of reduced expression of chondrocytes marker genes, inability to apply clinical treatment on a large scale, and low efficiency of osteoblast differentiation. Cell adhesion, beneficial to cell proliferation and differentiation, and the effect of improving the efficiency of induction of differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
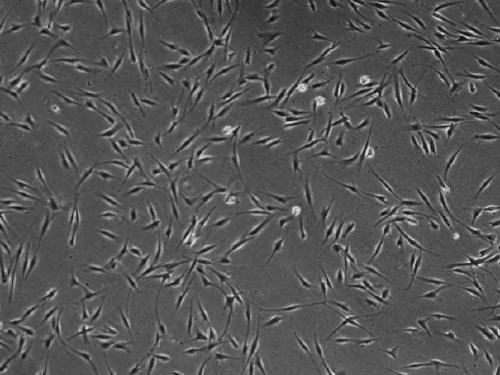
[0045] Example 1: Culture and passage of adipose stem cells
[0046] 1) Adipose tissue was extracted from the abdomen of volunteers, washed 3 times with PBS solution under sterile conditions, washed away red blood cells, and cut into fine particles with a scalpel;
[0047] 2) Add 0.1% collagenase solution 2 times the volume of adipose tissue, shake at 37°C for 2-3 hours, and digest;
[0048] 3) Collect the liquid after shaking and digesting, 1500rpm / 10min, discard the supernatant, and resuspend the pellet with low-sugar DMEM medium containing 10% FBS;
[0049] 4) Filtrate with a 200-mesh fine sieve to remove residual tissue impurities in the suspended cell liquid, and place the filtrate in a centrifuge tube;
[0050] 5) 1500rpm / 10min, discard the supernatant, resuspend the pellet in low-sugar DMEM medium containing 10% FBS, and culture in a 37°C, 5% CO2 incubator. These are primary cells. After 5 days, change the medium for the first time;
[0051] 6) When the confluence of ...
Embodiment 2
[0053] Example 2: Induction of differentiation of adipose stem cells into chondrocytes
[0054] (1) Preheating
[0055] Preheat 1.2% sodium alginate solution (alginate suspension prepared with 0.9% NaCl) and calcium chloride solution to 37°C.
[0056] (2) Preparation of adipose stem cell-sodium alginate suspension
[0057] The adipose stem cells (ADSCs) cultured in the T175 bottle to the P3 generation covered about 90% of the bottom of the bottle. The cells were washed with PBS solution for 3 times, and 2-3ml of 0.25% trypsin digestion solution was added to prepare the ADSCs suspension. The cell concentration was 4.5 ×10 6 a / ml;
[0058] The ADSCs suspension was thoroughly mixed with 1.2% sodium alginate solution at a volume ratio of 3:1 to make a cell concentration of 6.0×10 6 Adipose stem cell-sodium alginate suspension per ml, gently blow with a pipette to prevent the generation of air bubbles.
[0059] (3) Preparation of adipose stem cell-calcium alginate microbeads ...
Embodiment 3
[0073] Embodiment 3: MTT method detects cell proliferation activity
[0074] The adipose stem cells-calcium alginate microbeads of the above four groups on the 3d, 7d, and 14d were respectively inoculated into a 96-well plate, and 100 μl of cell suspension was added to each well, and the cell density was 5×10 4 a / m. There were 3 wells in each group, and a blank control group was set at the same time.
[0075] Add 10 μl of MTT solution (5 mg / ml) to each well, continue to incubate for 4 h in a 37°C, 5% CO2 incubator, carefully discard the supernatant in the well, add 150 μl of dimethyl sulfoxide (DMSO), shake for 10 min, and The absorbance of each well was measured at OD490nm with an immunodetector.
[0076] According to the MTT test, on the 3d, 7d, and 14d after induction, the OD values of the experimental groups B, C, and D were significantly higher than those of the control group A. On the 14th day, the OD value of the control group A was 0.26, and the OD value of the ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com