A kind of endo-type β-mannan hydrolase man01929 and its method and application of mutation into glycosyltransferase
A technology of mannanase and mutant enzymes, applied in the direction of transferase, hydrolase, glycosylase, etc., can solve the problems of little mechanism research, and achieve the effect of stable physical and chemical properties and wide pH tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Extraction of Genomic DNA from Pyrobacterium pyrobacter MY04 Strain
[0088] Pyrochrome bacterium MY04 was inoculated into liquid medium YT04, and cultured with shaking at 28°C and 200 rpm until its absorbance at 600 nm (OD 600 ) is 1.2; take 10mL of the cultured bacteria solution, and centrifuge at 4°C for 15min under the condition of 12,000×g (g, the gravitational constant of the earth) to collect the bacterial precipitate; Suspend the bacteria, centrifuge at 12,000x g, 4°C for 15 minutes, and collect the bacteria pellet.
[0089] The above-mentioned liquid medium YT04 has the following components per liter:
[0090] Tryptone 10g, yeast extract 5.0g, sodium chloride 30g, dissolved in water and adjusted to 1L, pH 7.2.
[0091] Add 6.0mL of lysozyme buffer solution to each tube to obtain about 7.0mL of bacterial solution, and add 280μL of lysozyme solution with a concentration of 20mg / mL to make the final concentration of lysozyme 800μg / mL; Place in an ice-water bath...
Embodiment 2
[0093] Genome Scanning and Sequence Analysis of Pyrobacterium pyrobacter MY04 Strain
[0094] Genomic DNA prepared in Example 1 was scanned and sequenced by Shanghai Meiji Biotechnology Co., Ltd. using pyrosequencing technology. The DNA sequencing results were analyzed with the online software of NCBI (National Center for Biotechnology Information, http: / / www.ncbi.nlm.nih.gov / ) website. The analysis software used on the NCBI website is Open Reading Frame Finder (ORF Finder, http: / / www.ncbi.nlm.nih.gov / gorf / gorf.html) and Basic Local Alignment Search Tool (BLAST, http: / / blast.ncbi.nlm.nih.gov / Blast.cgi).
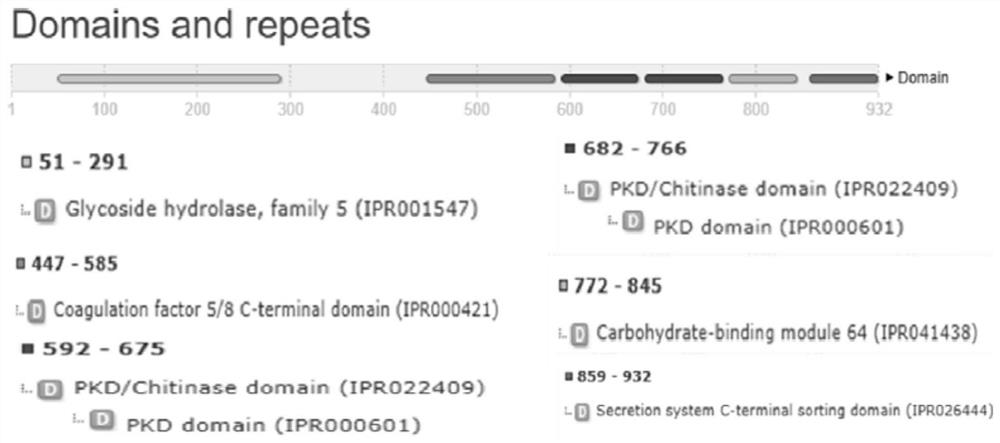
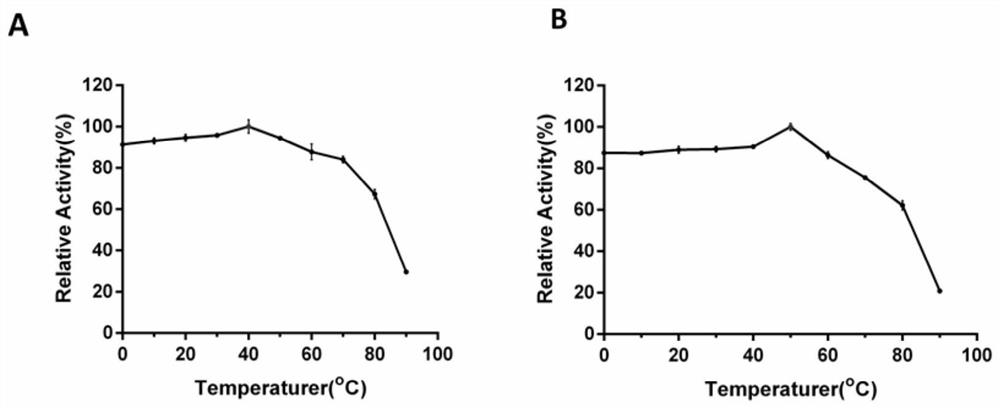
[0095] The results analyzed by the above-mentioned biological software show that the genomic DNA of the Pyrobacterium pyrobacterium MY04 strain carries a coding gene man01929 of β-mannanase, the coding region of the gene man01929 is 2799bp long, and the nucleotide sequence is shown in SEQ ID NO.1 . The β-mannanase Man01929 encoded by the gene man01929 contains a total of ...
Embodiment 3
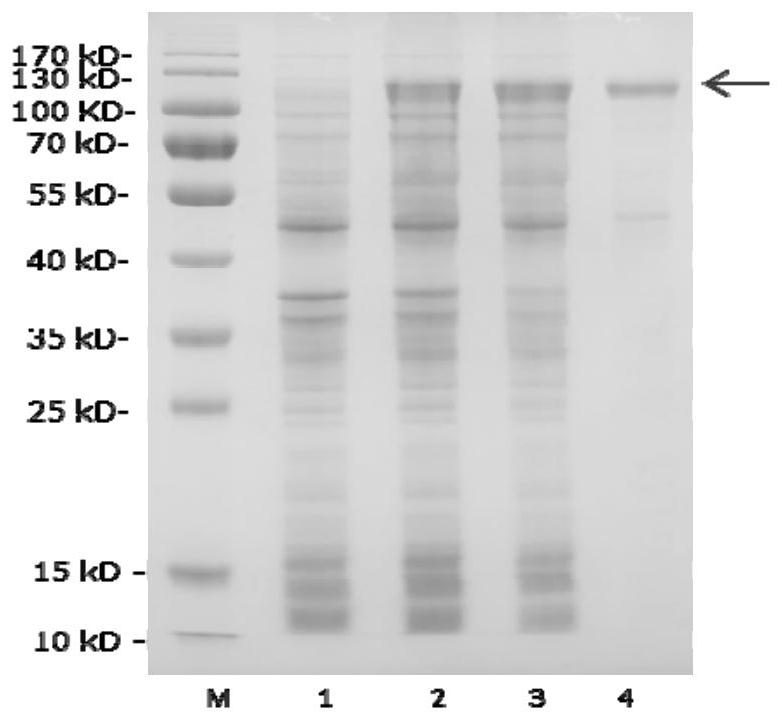
[0098] Recombinant expression of gene man01929 in Escherichia coli BL21 (DE3) bacterial strain:
[0099] Using the genomic DNA prepared in Example 1 as a template, PCR amplification was performed. The primer sequences are as follows:
[0100] forward primer for man01929 amplification
[0101] Man01929-F: 5'-gcg CATATG GCACTTTTTGCTCATGC-3';
[0102] reverse primer for man01929 amplification
[0103] Man01929-F: 5'-gcg CTCGAG TTGCTTGTAGATTCTCCTAAC-3';
[0104] The underlined mark of the forward primer is the specificity site of the restriction endonuclease Nde I, and the underlined mark of the reverse primer is the specificity site of the restriction endonuclease Xho I.
[0105] The high-fidelity DNA polymerase Prime STAR HS DNA Polymerase used was purchased from China Dalian Bao Biological Company, and the PCR reaction reagents used were operated according to the product instructions provided by the company.
[0106] PCR reaction system:
[0107] 2×Primer star GC buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com