Crystalline Li3OCl inorganic lithium ion conductor as well as preparation method and application thereof
An ion conductor, inorganic lithium technology, applied in the field of solid electrolyte preparation, can solve the problems of poor controllability of material morphology and electrochemical performance, harsh synthesis equipment and process conditions, etc., to improve lithium ion conductivity and control products. Morphology, the effect of high product conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Lithium hydroxide, lithium oxide and lithium chloride were prepared in a molar ratio (1.8 : 0.1 :1), ball milled to mix the three compounds evenly, and the mixture was placed in a vacuum tube furnace at a rate of 2°C / min to 300 ℃, and kept at 300℃ for 5h to obtain a molten precursor; uniformly disperse 0.2 mole of metal zinc powder in the molten precursor, and raise the temperature to 400℃ for 20h; then o Cool down to room temperature at a cooling rate of C / min. The resulting product is pulverized with a ball mill to obtain Zn-doped Li with a particle size distribution between 150-325 mesh. 3 OCl electrolyte material.
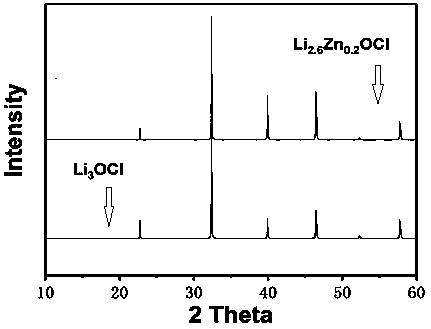
[0027] figure 1 Pure phase Li 3 OCl and embodiment 1 gained Zn-doped Li 3 The XRD collection of OCl; Whether can be seen in present embodiment 1 gained Zn-doped Li 3 OCl is crystalline
[0028] figure 2 For the obtained Zn doped Li of embodiment 1 3 The microscopic morphology of OCl, as can be seen from the figure, the resulting Zn-doped Li 3 ...
Embodiment 2
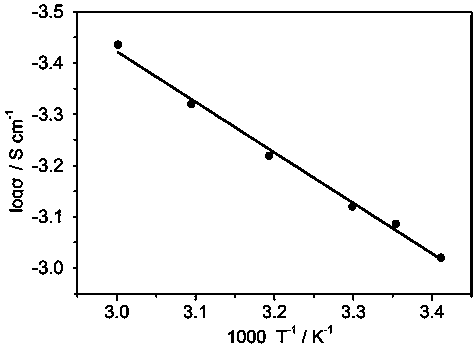
[0031] Lithium hydroxide, lithium oxide and lithium chloride were prepared in a molar ratio (1.8 : 0.1 :1), ball milled to mix the three compounds evenly, and the mixture was placed in a vacuum tube furnace at a rate of 2°C / min to 300 ℃, and held at 300°C for 5 hours to obtain a molten precursor; 0.05 mole metal zinc powder was uniformly dispersed in the molten precursor, and the temperature was raised to 400°C for 20 hours; o Cool down to room temperature at a cooling rate of C / min. The resulting product is pulverized with a ball mill to obtain Zn-doped Li with a particle size distribution between 150-325 mesh. 3 OCl electrolyte material. According to the above method, a symmetrical battery was prepared and the electrochemical performance was tested. The results showed that: 0.05 moles of Zn doped Li 3 The room temperature ionic conductivity of OCl is 0.95×10 -3 S / cm.
Embodiment 3
[0033] Prepare lithium hydroxide, lithium oxide and lithium chloride in a molar ratio (1.8 : 0.1 :1), ball mill to mix the three compounds evenly, put the mixture in a vacuum tube furnace, and raise it to 300 °C at a rate of 2 °C / min ℃, and held at 300°C for 5 hours to obtain a molten precursor; 0.1 mole metal zinc powder was uniformly dispersed in the molten precursor, and the temperature was raised to 400°C for 20 hours; o Cool down to room temperature at a cooling rate of C / min. The resulting product is pulverized with a ball mill to obtain Zn-doped Li with a particle size distribution between 150-325 mesh. 3 OCl electrolyte material. According to the above method, a symmetrical battery was prepared and the electrochemical performance was tested. The results showed that: 0.1 mole Zn doped Li 3 The room temperature ionic conductivity of OCl is 1.0×10 -3 S / cm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com