Synthesis method of triarylmethane derivative
A technology of triarylmethane and synthesis method, which is applied in the field of synthesis of triarylmethane derivatives, can solve the problems of limited reaction substrate range, poor regioselectivity, severe reaction conditions, etc., and achieves good stereoselectivity and reaction time. Effects with short, simple composition steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
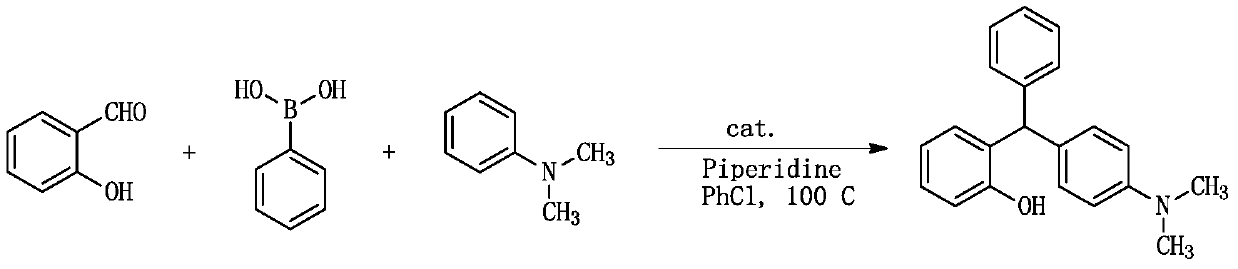
[0036] A kind of synthetic method of triarylmethane derivative ((4-dimethylaminophenyl) (2-hydroxyphenyl) (phenyl) methane), comprises the following steps:
[0037]
[0038] 1.0mmol (0.122g) salicylaldehyde, 1.0mmol (0.122g) phenylboronic acid, 1.2mmol (0.102g) piperidine, 1.2mmol (0.145g) N, N-dimethylaniline and 0.5mmol (0.127g) ) catalyst (catalyst is I 2 ) into a 10ml dry round-bottomed flask, then add 1ml chlorobenzene as a solvent, heat to reflux at 100°C, monitor the reaction by TLC, after the reaction is complete, cool to room temperature, add 30ml ethyl acetate to the reaction system, and use 20ml Washed with water and 10ml saturated brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and after a large amount of solvent was evaporated under reduced pressure, the residue was separated by column chromatography, using n-hexane and ethyl acetate as eluents, and the solvent Evaporated and dried to obtain the target compound, the reaction yield w...
Embodiment 2
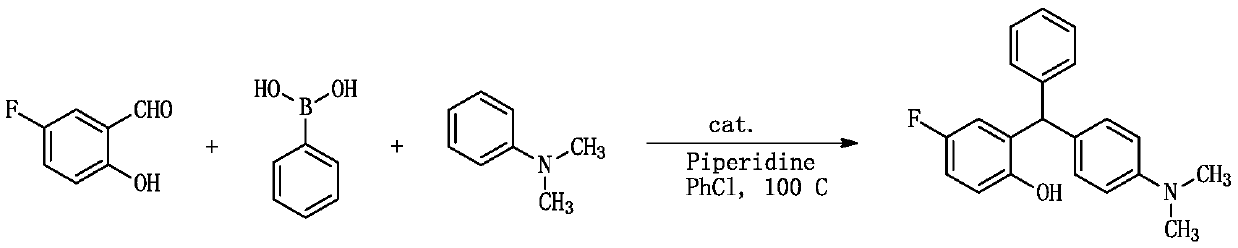
[0040] A kind of synthetic method of triarylmethane derivative ((4-dimethylaminophenyl) (2-hydroxyl-4 fluorophenyl) (phenyl) methane), comprises the following steps:
[0041]
[0042] 1.0mmol (0.140g) 5-fluoro salicylaldehyde, 1.0mmol (0.122g) phenylboronic acid, 1.2mmol (0.102g) piperidine, 1.2mmol (0.145g) N,N-dimethylaniline and 0.5mmol (0.127g) catalyst (catalyst is I 2 ) into a 10ml dry round-bottomed flask, then add 1ml chlorobenzene as a solvent, heat to reflux at 100°C, monitor the reaction by TLC, after the reaction is complete, cool to room temperature, add 30ml ethyl acetate to the reaction system, and use 20ml Washed with water and 10ml saturated brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and after a large amount of solvent was evaporated under reduced pressure, the residue was separated by column chromatography, using n-hexane and ethyl acetate as eluents, and the solvent Evaporated and dried to obtain the target compound, the ...
Embodiment 3
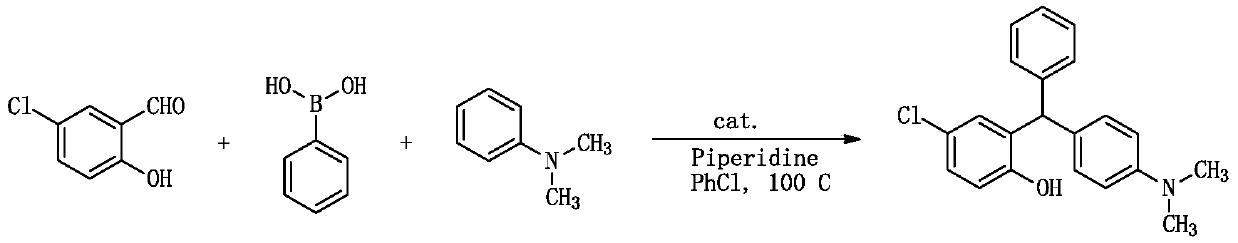
[0044] A kind of synthetic method of triarylmethane derivative ((4-dimethylaminophenyl) (2-hydroxyl-4 chlorophenyl) (phenyl) methane), comprises the following steps:
[0045]
[0046] 1.0mmol (0.156g) 5-chloro salicylaldehyde, 1.0mmol (0.122g) phenylboronic acid, 1.2mmol (0.102g) piperidine, 1.2mmol (0.145g) N, N-dimethylaniline and 0.5mmol (0.127g) catalyst (catalyst is I 2 ) into a 10ml dry round-bottomed flask, then add 1ml chlorobenzene as a solvent, heat to reflux at 100°C, monitor the reaction by TLC, after the reaction is complete, cool to room temperature, add 30ml ethyl acetate to the reaction system, and use 20ml Washed with water and 10ml saturated brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and after a large amount of solvent was evaporated under reduced pressure, the residue was separated by column chromatography, using n-hexane and ethyl acetate as eluents, and the solvent Evaporated and dried to obtain the target compound, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com