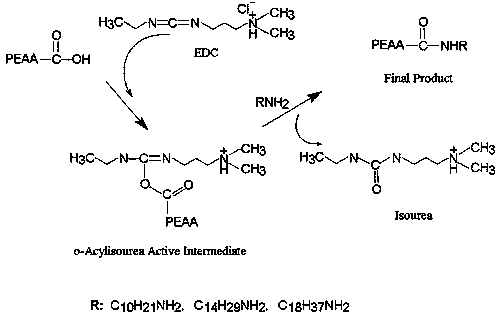
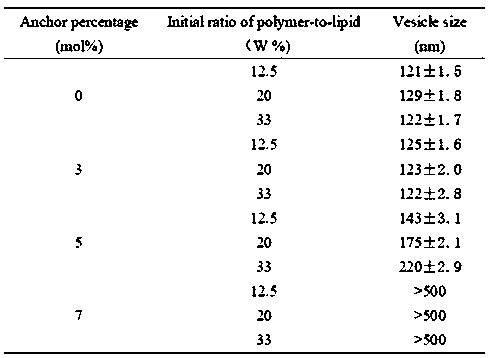
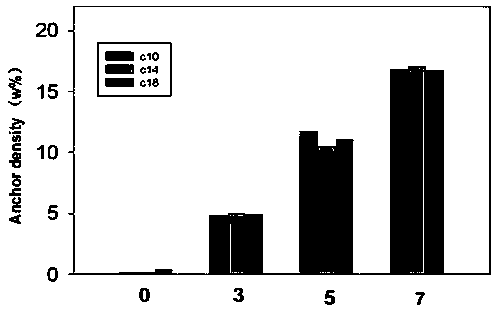
Intelligent macromolecular amphoteric lipid material as well as synthesis method and application thereof in complex drug delivery preparation
An intelligent polymer and lipid material technology, applied in the field of intelligent liposomes, can solve the problems of inability to release drugs and function, and improve the effect of treating diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1.1 Liposome preparation
[0035]Prepare large unilamellar liposomes according to the extrusion method published by Hope et al. Take an appropriate amount of phospholipid mixture (lecithin / cholesterol, 55:45 molar ratio) and isotope-labeled cholesteryl ester [3H]-cholesteryl hexadecyl ether (radioactive (radioactivity is 1.33Ci / 4mol)) was co-dissolved in chloroform / methanol, added nitrogen gas in the test tube to dry to form a uniform mixed phospholipid film, and dried overnight in vacuum. Add HBS buffer (20 mmol / L Hepes, 150 mmol / L NaCl, pH 7.5) or HBS buffer containing calcein (100 mM) into the test tube containing the phospholipid membrane, shake rapidly to hydrate and dissolve the phospholipid membrane. The obtained multilamellar vesicles (MLVs) were quick-frozen in liquid nitrogen and then hydrolyzed in 55°C water for 5 rounds. Lamellar liposomes were repeatedly extruded through a double-layer 100 nm pore membrane (Nucleopore) for 10 times to obtain the required l...
Embodiment 2
[0056] 2.1 Drugs and reagents
[0057] Fluorescently labeled phospholipids N-(7-nitro2,1,3-benzoxadiazol-4-yl) phosphatidy lethanolamine (NBD-PE), rhodamine phosphatidy lethanolamine (Rh-PE ), distearoylphosphatidylcholine (DSPC), dipalmitoyl phosphatidylcholine (DPPC) and dimiristoyl phosphatidylg Lycerol (DMPG) was purchased from (Avanti polar lipids company in the United States), lecithin (EPC, 98% purity) and cholesterol (Chol, 98% purity) were provided by Xi’an Libang Pharmaceutical Co., Ltd., and MH medium was a product of Gibco Company in the United States. Poly(2-ethylacrylic acid) (molecular weight: 20,000) was provided by Fu Jingguo, a researcher at the Institute of Chemistry, Henan Academy of Sciences, and the rest of the reagents were of domestic analytical grade.
[0058] 2.2 Drugs and reagents
[0059] Extruder (Canada Lipex Biomembranes Company), LS-50B Fluorescence Spectrophotometer (U.S. Perkin-Elmer Company), Nicomp 370 Laser Scattering Particle Size Analyze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com