Preparation method of iohexol impurity I
A technology of iohexol and impurities, applied in the field of preparation of iohexol impurity I, can solve the problem of high cost, and achieve the effects of low cost, easily available raw materials, and controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
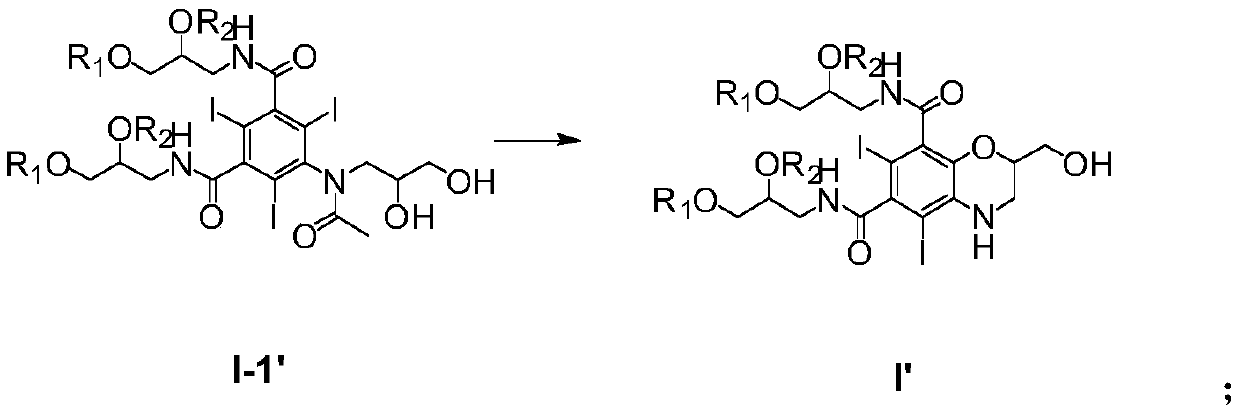
[0023] The present invention provides a method for preparing iohexol impurity I, comprising the following steps: (1) using iohexol I-1' as a raw material, an intramolecular coupling reaction occurs under alkaline conditions to form compound I', The synthetic route is
[0024]
[0025] where the R 1 and R 2 Each is independently a hydroxyl protecting group, and the hydroxyl protecting group is selected from an alkyl group, a cycloalkyl group, an acyl group, a silyl group, an aryl group and a heteroaryl group; or, R 1 and R 2 together form a heterocyclic group;
[0026] (2) Removal of R 1 and R 2 Hydroxyl protecting group, to form iohexol impurity I, synthetic route is
[0027]
[0028] Preferably, the base of the alkaline condition is at least one of alkali metal alcoholate, alkali metal carbonate, alkali metal hydroxide, alkaline earth metal carbonate and alkaline earth metal hydroxide, preferably tert-butanol At least one of sodium, potassium tert-butoxide, sodiu...
Embodiment 1
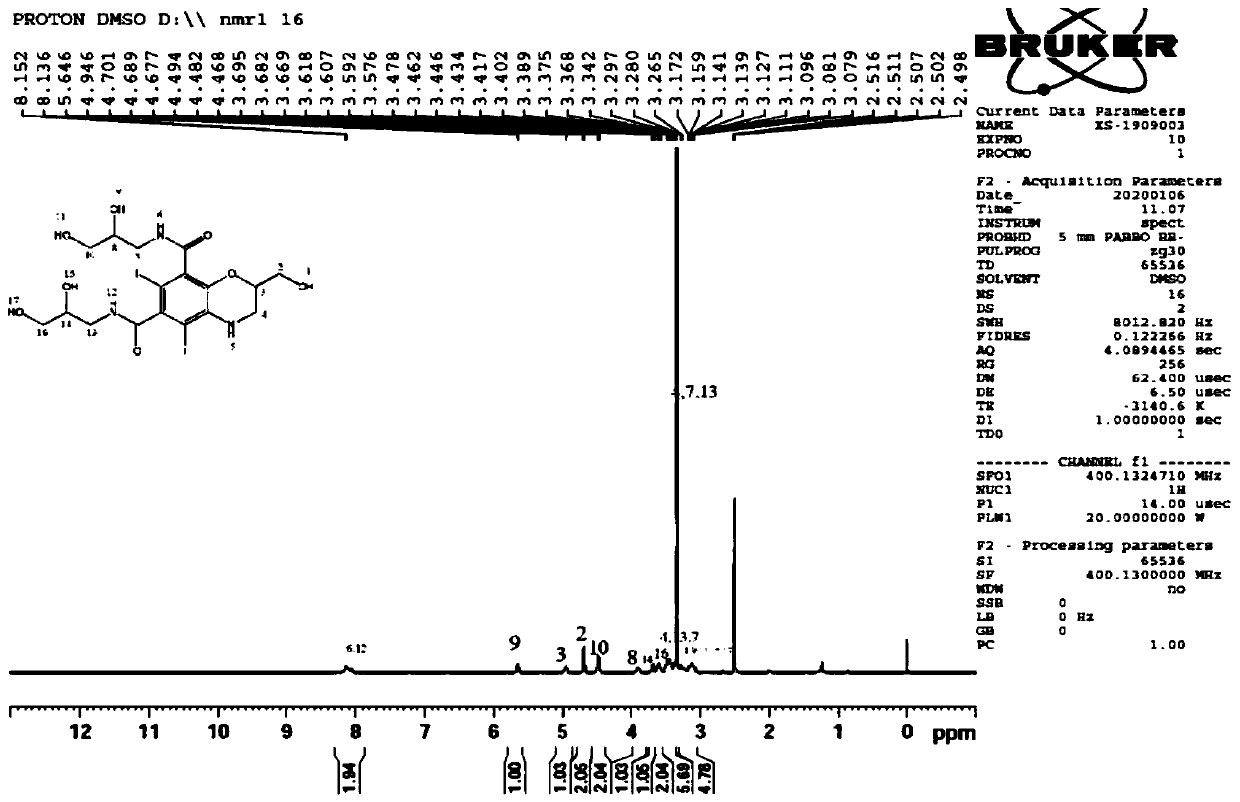
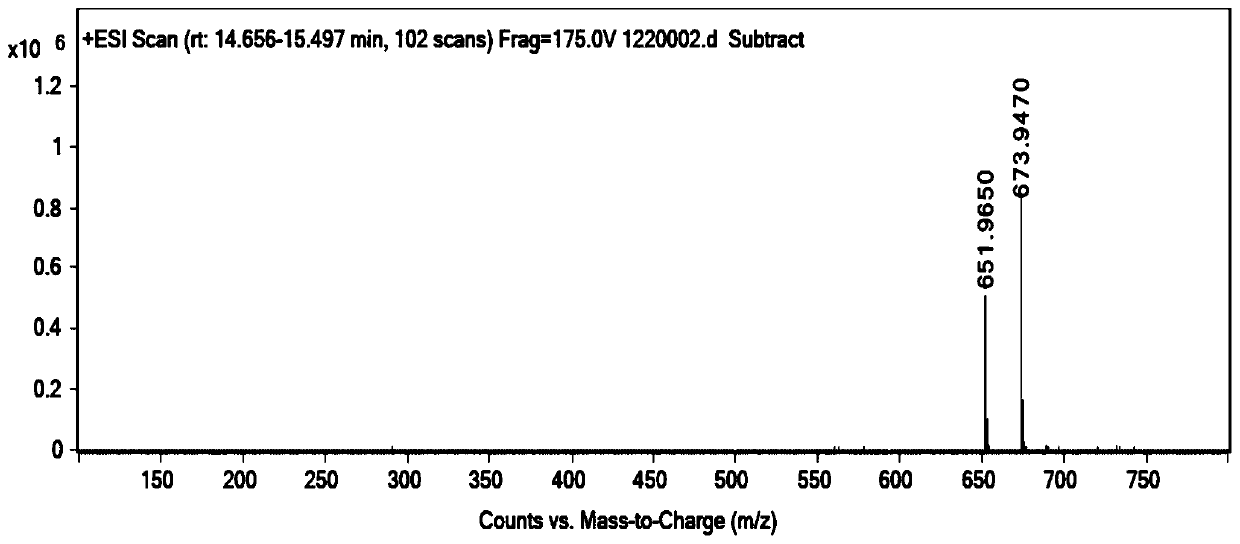
[0040]Under nitrogen protection, add iohexol (8.2g, 0.01mol) and sodium hydroxide aqueous solution (12.0g, 0.03mol) in the reaction flask of 100ml, stir reaction at room temperature for 12 hours, TLC detects raw material iohexol reaction completely, Adjust the pH to 5 with hydrochloric acid, concentrate, add ethyl acetate to dissolve, filter, and the filtrate is subjected to column chromatography to obtain 5.2 g of the product, with a yield of 80%, ESI (+) MSm / z 651.96 (M+H).
Embodiment 2
[0042] Under the protection of nitrogen, add iohexol (8.2g, 0.01mol) and sodium hydroxide aqueous solution (10.0g, 0.03mol) to a 100ml reaction flask, heat up to 100°C and stir for 12 hours, TLC detects the raw material iohexol After the reaction was complete, adjust the pH to 5 with hydrochloric acid, concentrate, add ethyl acetate to dissolve, filter, and the filtrate was subjected to column chromatography to obtain 5.2 g of the product, with a yield of 80%, ESI (+) MSm / z 651.96 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com