Application of sulfonyl type compounds as chlorination reagent
A technology of sulfonyl-type and chlorination reagents, which is applied in the field of sulfonyl-type compounds as chlorination reagents, which can solve the problems of unfavorable industrial production, expensive reagents, and inconvenient operation, and achieve the effect of reducing post-treatment and three-waste treatment steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0026] Preparation Example 1: Preparation of Compound (a)
[0027]
[0028] Step 1: Add 20g (0.435mol) of ethanol to a 250mL round-bottomed flask, lower the temperature of the system to 0 to -5°C, then slowly add 58.7g (0.435mol) of sulfonyl chloride dropwise to ethanol, and with Stir vigorously, control the dropping speed, and ensure that the temperature of the system is maintained at 0 to -5°C during the entire dropping process, the reaction releases hydrogen chloride gas, and the tail gas is absorbed by water to obtain hydrochloric acid with a mass concentration of 10-15%;
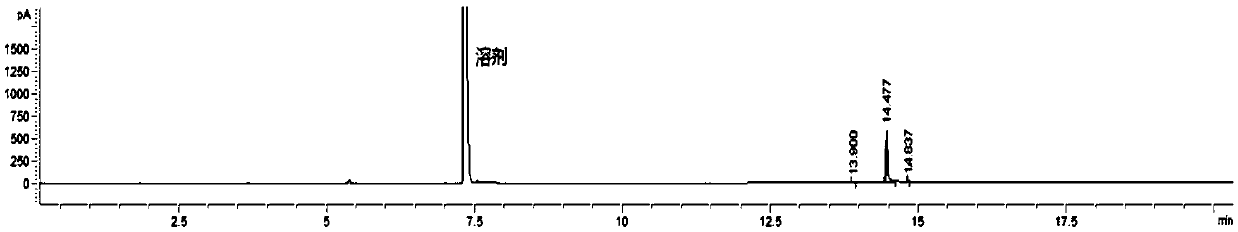
[0029] Step 2: After the dropwise addition, a small amount of hydrogen chloride and unreacted ethanol dissolved in the reaction system were removed by distillation under reduced pressure to obtain 58.7 g of a light yellow liquid, which was compound (a). After carrying out chlorination determination with the substrate, The effective chlorination reagent content is about 90%.
[0030] 1 H-NMR (400MH,...
preparation Embodiment 2
[0031] Preparation Example 2: Preparation of Compound (b)
[0032]
[0033] Step 1: Add 47g (0.435mol) benzyl alcohol and 50mL methylene chloride to a 250mL round bottom flask, lower the system temperature to 0 to -5°C, then slowly add 58.7g (0.435mol) sulfonyl chloride dropwise To the system, and accompanied by vigorous stirring, control the addition rate to ensure that the temperature of the system is maintained at 0 to -5°C during the entire addition process, the reaction releases hydrogen chloride gas, and the tail gas is absorbed by water to obtain a mass concentration of 10-15 % hydrochloric acid;
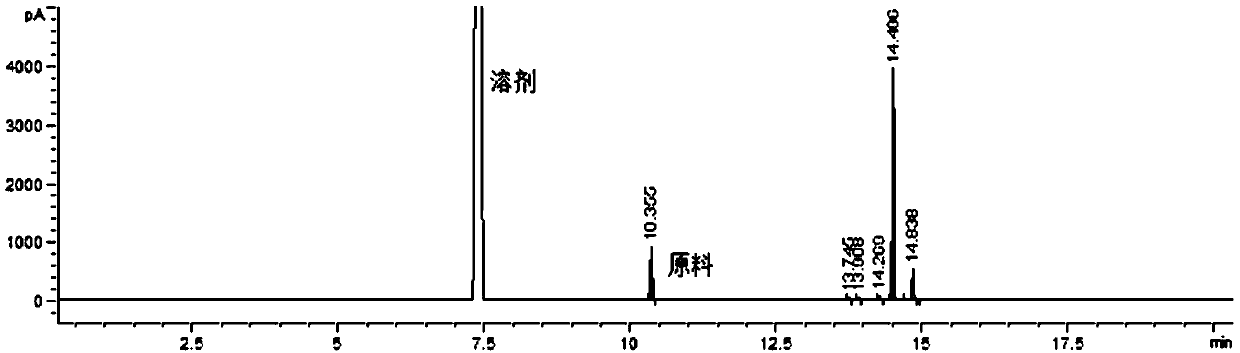
[0034] Step 2: after the dropwise addition, a small amount of hydrogen chloride and solvent dichloromethane dissolved in the reaction system were removed by distillation under reduced pressure to obtain 84.5g of yellow oily liquid, which is compound (b). After carrying out chlorination measurement with the substrate, effective The content of chlorination reagent is about ...
Embodiment 1
[0037] The compound (a) prepared in Preparation Example 1 is used as a chlorination reagent to chlorinate 1-chloro-1'-acetylcyclopropane, and the reaction formula is:
[0038]
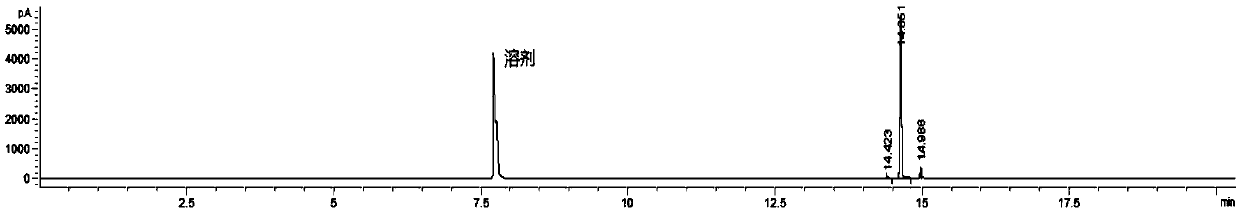
[0039] Add 50g of 1-chloro-1'-acetylcyclopropane and 50ml of dichloromethane into a 250ml three-necked round-bottomed flask, put it in a 0°C cold bath to lower the temperature, and add 67g of compound (a) (containing 90% of effective chlorination reagent) dropwise under stirring. %), dropwise temperature control between 0 and -5°C, dropwise time of 3 hours, after addition, continue to react for 1 hour, at room temperature, remove the organic solvent under reduced pressure, and raise the temperature to 60°C, continue to reduce pressure Residual sulfur dioxide in the reaction system was distilled off to obtain 64 g of a light yellow transparent oily liquid, which was confirmed to be 1-chloro-1-chloroacetylcyclopropane with a purity of 96.5% by GC-MS and H-NMR. The product can be used directly in the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com