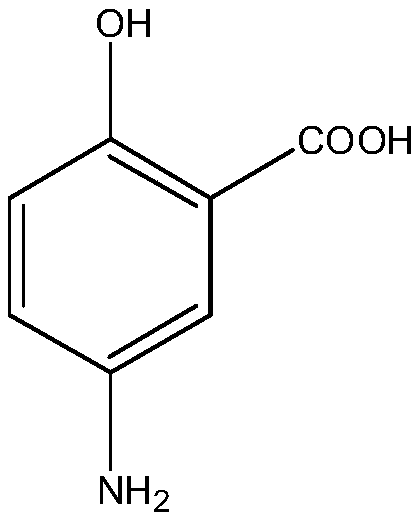
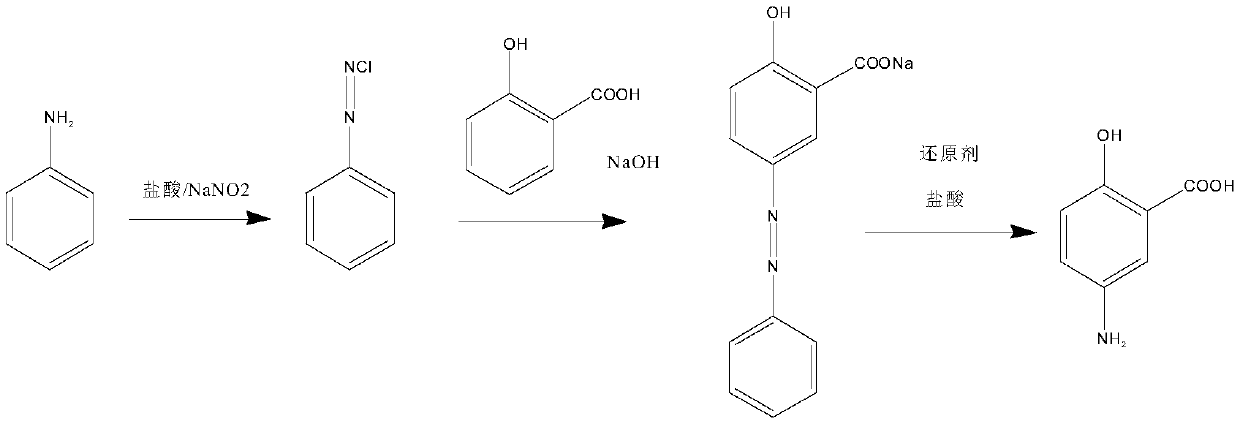
One-pot catalytic synthesis method of 5-aminosalicylic acid
A technology of aminosalicylic acid and synthesis method, which is applied in the field of medicine, can solve the problems of high requirements for reaction equipment, large amount of wastewater, low selectivity, etc., achieve obvious economic and social benefits, reduce high-salt wastewater discharge, and reduce environmental protection cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of diazonium
[0023] Put 9.31g of aniline, 50mL of water, and 22.80g of concentrated hydrochloric acid into the reaction flask in sequence, then stir, and cool down to 0-5°C. Control the temperature at 0-5°C, add sodium nitrite / water (7.04g / 20mL) solution dropwise, after the dropwise addition, use potassium iodide starch test paper to detect, the detection shows blue, and the reaction is terminated to obtain diazonium solution.
Embodiment 2
[0024] Embodiment 2: azo compound synthesis
[0025] Put 13.80g of salicylic acid, 200mL of water, and 45.00g of 30% sodium hydroxide into the reaction flask in turn. After dissolving, cool down to -5~5°C; solution. After the dropwise addition, keep the temperature at 0-5°C for 3-3.5 hours, take a sample, and perform HPLC detection. If the salicylic acid is less than 1.0%, the reaction is completed, and the reaction solution of the conjugate is obtained.
Embodiment 3
[0026] Example 3: Raney nickel reduction
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com