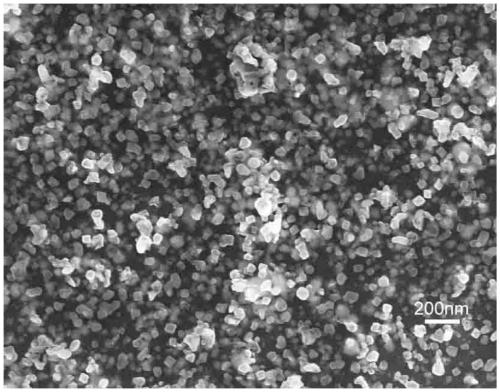
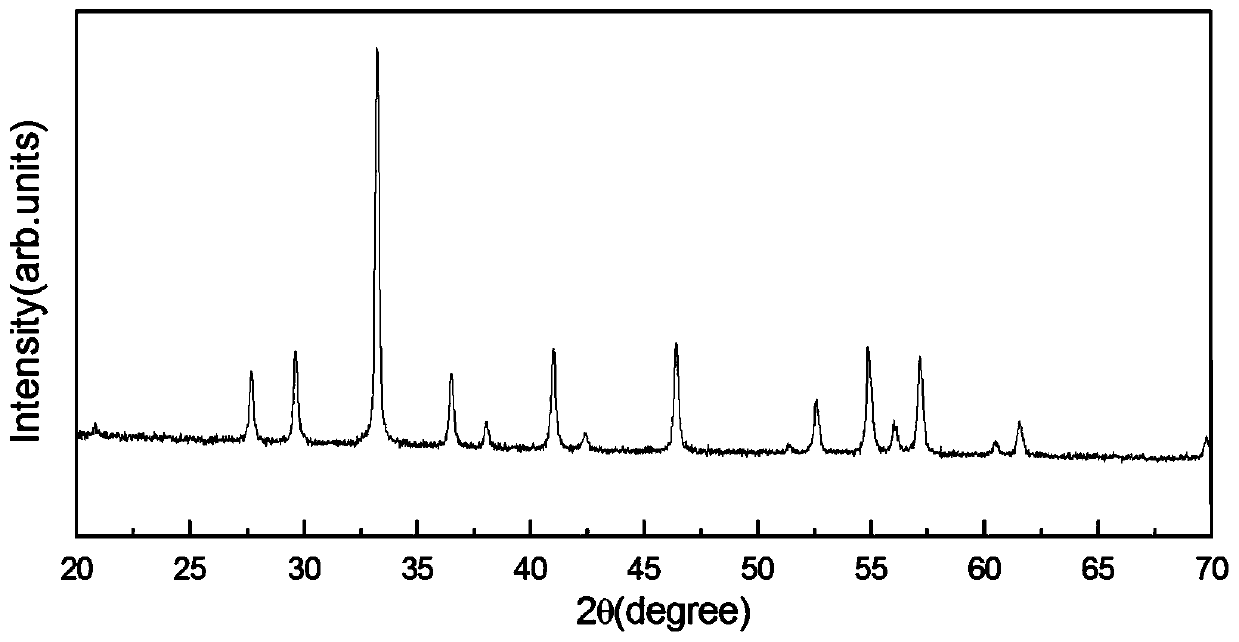
Method for preparing yttrium aluminum garnet powder
A technology of yttrium aluminum garnet and garnet powder, which is applied in the field of polycrystalline ceramic powder preparation, can solve problems such as difficulty in obtaining yttrium aluminum garnet polycrystalline powder, difficulty in obtaining uniformly dispersed YAG powder, and affecting the purity of YAG products. , to achieve the effect of uniform composition, less pollution and less consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0026] (1) Reaction raw material Y(NO 3 ) 3 ·6H 2 O and aluminum isopropoxide were thoroughly dried at 160°C. According to chemical formula Y 3 Al 5 o 12 , Weighed 10.814g yttrium nitrate hexahydrate, 9.600g aluminum isopropoxide and 0.6g NaOH respectively.
[0027] (2) Dissolve the weighed yttrium nitrate hexahydrate in 30ml of deionized water, dissolve NaOH in 30ml of deionized water, and dissolve aluminum isopropoxide in 20ml of absolute ethanol to prepare corresponding solutions.
[0028] (3) At room temperature, slowly add the NaOH solution dropwise into the yttrium nitrate solution while stirring until the pH value of the solution becomes 12, then stop the titration to obtain a suspension of the yttrium hydroxide precipitation precursor.
[0029] (4) Centrifuge the obtained precipitation precursor suspension at high speed, slowly suck out the supernatant with a syringe or a straw, then add deionized water to wash the lower filter cake, and then centrifuge and wash ...
Embodiment 2
[0036] (1) Reaction raw material Y(NO 3 ) 3 ·6H 2 O and aluminum isopropoxide were thoroughly dried at 140°C. According to chemical formula Y 3 Al 5 o 12 , respectively weighed 5.632g of yttrium nitrate hexahydrate, 5g of aluminum isopropoxide and 0.3g of NaOH.
[0037] (2) Dissolve the weighed yttrium nitrate hexahydrate in 15ml of deionized water, dissolve NaOH in 30ml of deionized water, and dissolve aluminum isopropoxide in 25ml of absolute ethanol to prepare corresponding solutions.
[0038] (3) At room temperature, slowly add the NaOH solution dropwise into the yttrium nitrate solution while stirring until the pH value of the solution becomes 12, then stop the titration to obtain a suspension of the yttrium hydroxide precipitation precursor.
[0039] (4) Centrifuge the obtained precipitation precursor suspension at high speed, slowly suck out the supernatant with a syringe or a straw, then add deionized water to wash the lower filter cake, and then centrifuge and was...
Embodiment 3
[0045] (1) Reaction raw material Y(NO 3 ) 3 ·6H 2 O and aluminum isopropoxide were thoroughly dried at 120°C. According to chemical formula Y 3 al 5 o 12 , respectively weighed 4.055g of yttrium nitrate hexahydrate, 3.600g of aluminum isopropoxide and 0.6g of NaOH.
[0046] (2) Dissolve the weighed yttrium nitrate hexahydrate in 20ml of deionized water, dissolve NaOH in 30ml of deionized water, and dissolve aluminum isopropoxide in 20ml of absolute ethanol to prepare corresponding solutions.
[0047] (3) At room temperature, slowly add the NaOH solution dropwise into the yttrium nitrate solution while stirring until the pH value of the solution becomes 12, then stop the titration to obtain a suspension of the yttrium hydroxide precipitation precursor.
[0048] (4) Centrifuge the obtained precipitation precursor suspension at high speed, slowly suck out the supernatant with a syringe or a straw, then add deionized water to wash the lower filter cake, and then centrifuge a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com