Preparation method of difluoromethyl aromatic hydrocarbon compound
A technology of difluoromethyl aromatic hydrocarbons and compounds, which is applied in the field of preparation of difluoromethyl aromatic hydrocarbons, can solve the problems of difficulty in preparing difluoromethyl compounds, inability to obtain a single product, incompatibility, etc., and achieve good functional group compatibility Sexuality, mild conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
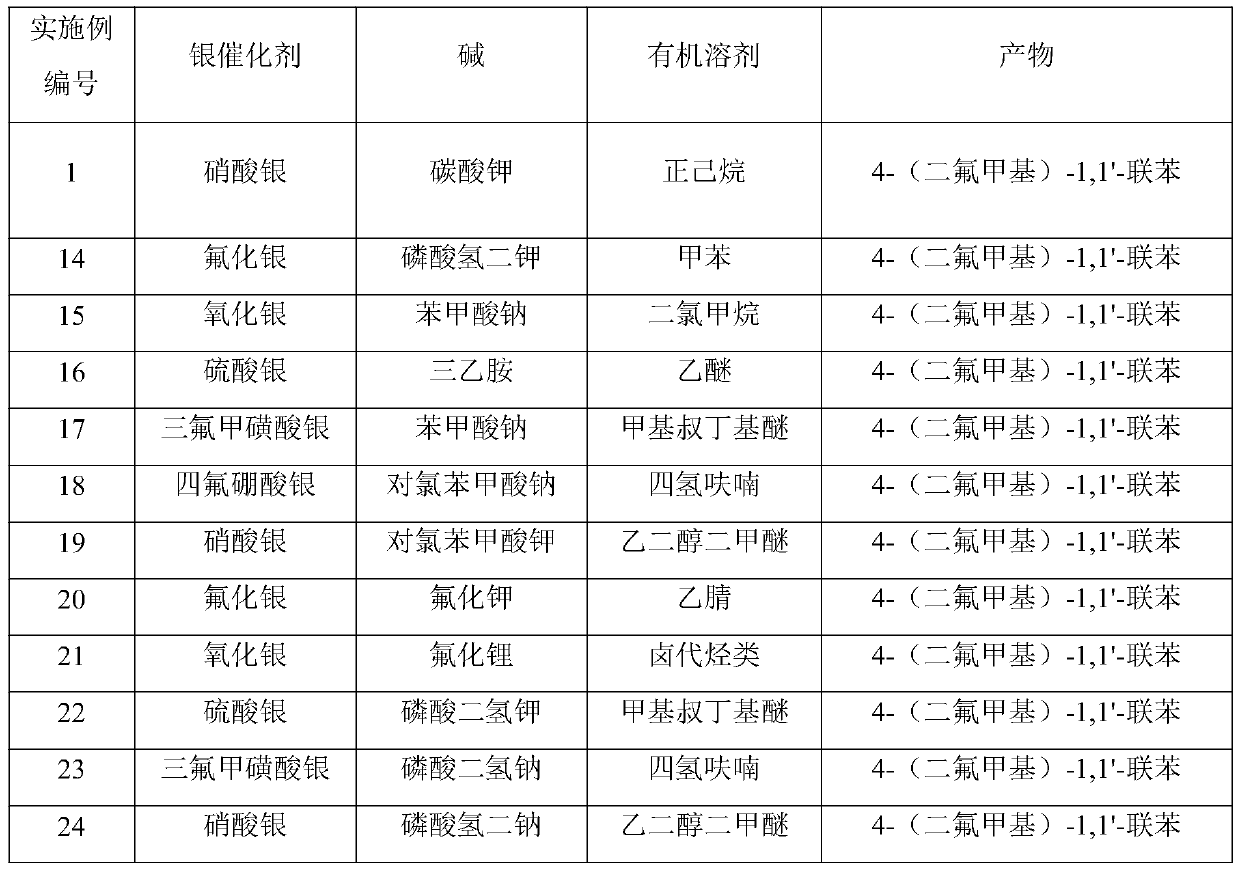
Embodiment 1
[0029] Example 1 Synthesis of 4-(difluoromethyl)-1,1'-biphenyl
[0030] (1) 2-([1,1'-biphenyl]-4-yl)malonic acid (51.2mg, 0.2mmol, 1.0equiv.), silver nitrate (10.2mg, 0.06mmol, 0.3equiv.), Potassium carbonate (82.9mg, 0.6mmol, 3.0equiv.) and selective fluorine reagent Selectfluor (566.8mg, 1.6mmol, 8.0equiv.) were added to the reaction tube, the reaction system was replaced by nitrogen, and n-hexane / water=1 : 1 total 4mL, react at 25°C for 12h;
[0031] (2) Add 2 mL of 3M hydrochloric acid to the reaction system to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and spin-dry the organic phase. The crude product is purified on a preparative silica gel plate (petroleum ether / ethyl acetate=70:1) 32.7 mg of the target product 4-(difluoromethyl)-1,1'-biphenyl was obtained as a white solid with a yield of 80%.
[0032] 1 H NMR (400MHz, CDCl 3 ): δ7.67(d, J=8.2Hz, 2H), 7.58(t, J=7.2Hz, 4H)...
Embodiment 2
[0035] Example 2 Synthesis of 3-(difluoromethyl)-1,1'-biphenyl
[0036] (1) 2-([[1,1'-biphenyl]-3-yl)malonic acid (51.2mg, 0.2mmol, 1.0equiv.), silver nitrate (10.2mg, 0.06mmol, 0.3equiv.) , potassium carbonate (82.9mg, 0.6mmol, 3.0equiv.) and selective fluorine reagent Selectfluor II (566.8mg, 1.6mmol, 8.0equiv.) were added to the reaction tube, the reaction system was replaced by nitrogen, and n-hexane / water was added =1:1 total 4mL, react at 25°C for 12h;
[0037](2) Add 2 mL of 3M hydrochloric acid to the reaction system to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and spin-dry the organic phase. The crude product is purified on a preparative silica gel plate (petroleum ether / ethyl acetate=70:1) 24.5 mg of the target product 3-(difluoromethyl)-1,1'-biphenyl was obtained as a white solid with a yield of 60%.
[0038] 1 H NMR (400MHz, CDCl 3 ): δ7.75–7.66(m,2H),7.63–7.57(m,2...
Embodiment 3
[0041] The synthesis of embodiment 3 difluoromethylbenzene
[0042] (1) 2-phenylmalonic acid (36.0mg, 0.2mmol, 1.0equiv.), silver nitrate (10.2mg, 0.06mmol, 0.3equiv.), potassium carbonate (82.9mg, 0.6mmol, 3.0equiv.) And selective fluorine reagent (566.8mg, 1.6mmol, 8.0equiv.) was added to the reaction tube, the reaction system was replaced by nitrogen, a total of 4mL was added with n-hexane / water=1:1, and the reaction was carried out at 25°C for 12h;
[0043] (2) Using 4-fluorobiphenyl as an internal standard, the reaction system was passed 19 F NMR assay yield is 66%, because the product is volatile, by 19 F NMR and GC / MS identified the product as difluoromethylbenzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com