Production process of dimethyl phthalate
A dimethyl phthalate and production process technology, applied in the field of dimethyl phthalate production process, can solve the problems of high product odor, unfavorable energy saving and emission reduction, high energy consumption, etc., and achieve the goal of reducing steam consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
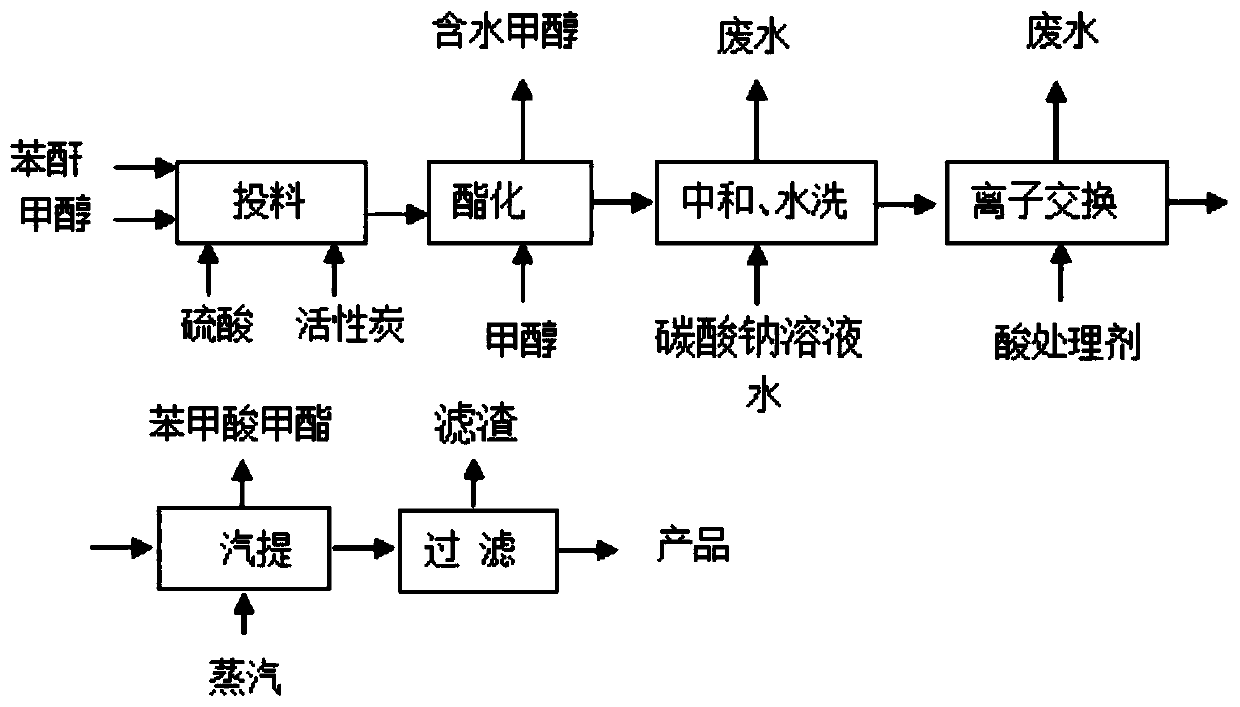
[0032] see figure 1 , a production process of dimethyl phthalate, the production process comprising:
[0033] Step 1), esterification reaction:
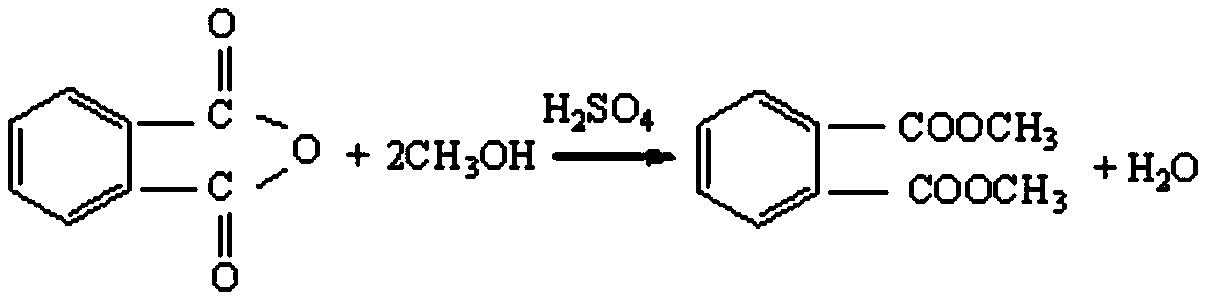
[0034] Put phthalic anhydride and methanol into the esterification kettle with a molar ratio of 1:2, then add catalyst sulfuric acid with phthalic anhydride quality of 2‰, raise the temperature to 80°C for reflux reaction for 2 hours, raise the vacuum in the kettle to above 0.08MPa, and slowly exit the kettle Excess methanol in the tank and the water generated by the reaction until the temperature in the tank is 120°C, then add methanol to the tank while dehydrating at a temperature of 120°C under a vacuum degree of less than 0.01MPa, and carry out esterification until the acid value of the material is less than 4mgKOH / g, the esterification reaction time is 15 hours, and then the excess methanol is removed under a vacuum greater than 0.09MPa, and the dealcoholization temperature is 130°C to obtain a crude ester, which contains 98.5...
Embodiment 2
[0045] see figure 1 , a production process of dimethyl phthalate, the production process comprising:
[0046] Step 1), esterification reaction:
[0047] Put phthalic anhydride and methanol into the esterification kettle with a molar ratio of 1:2, then add catalyst sulfuric acid with phthalic anhydride quality of 2.5‰, raise the temperature to 95°C and reflux for 2.5 hours, raise the vacuum in the kettle to above 0.08MPa, and slowly exit the kettle Excess methanol in the tank and water generated by the reaction until the temperature in the tank is 128°C, and then add methanol to the tank while dehydrating at a temperature of 125°C under a vacuum degree of less than 0.01MPa, and carry out esterification reaction until the acid value of the material is less than 4mgKOH / g, the esterification reaction time is 13 hours, and then the excess methanol is removed under a vacuum greater than 0.09MPa, and the dealcoholization temperature is 136°C to obtain a crude ester, which contains ...
Embodiment 3
[0058] see figure 1 , a production process of dimethyl phthalate, the production process comprising:
[0059] Step 1), esterification reaction:
[0060] Put phthalic anhydride and methanol into the esterification kettle at a molar ratio of 1:2, then add catalyst sulfuric acid with phthalic anhydride quality of 3‰, heat up to 100°C and reflux for 3 hours, raise the vacuum in the kettle to above 0.08MPa, and slowly exit the kettle Excess methanol in the tank and water generated by the reaction until the temperature in the tank is 130°C, then dehydration is carried out while adding methanol to the tank at a temperature of 130°C under a vacuum degree of less than 0.01MPa, and the acid value of the material is less than 4mgKOH / g, the esterification reaction time is 12 hours, and then the excess methanol is removed under a vacuum greater than 0.09MPa, and the dealcoholization temperature is 140°C to obtain a crude ester, which contains 98.5-99% of dimethyl phthalate and benzoic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com