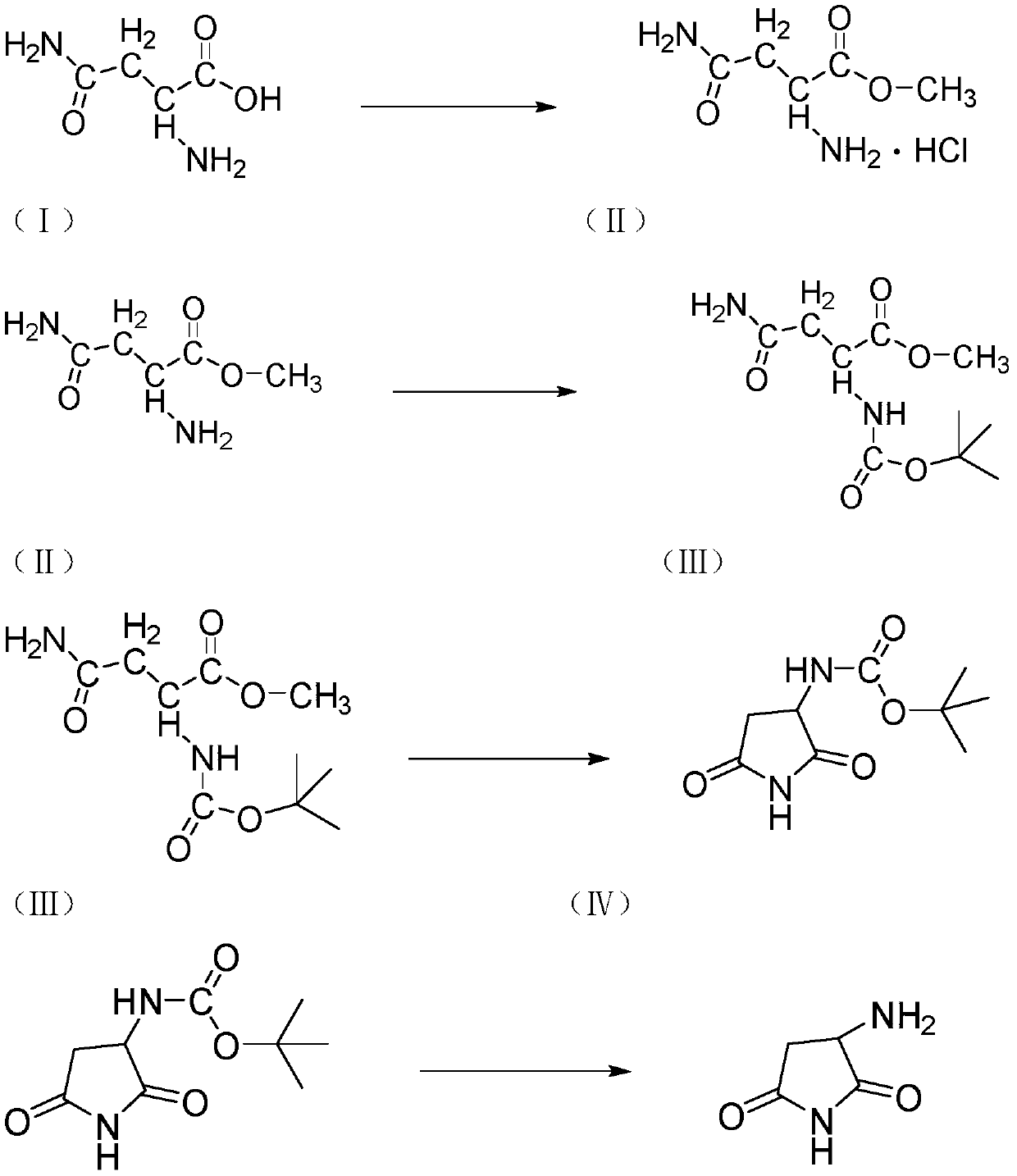
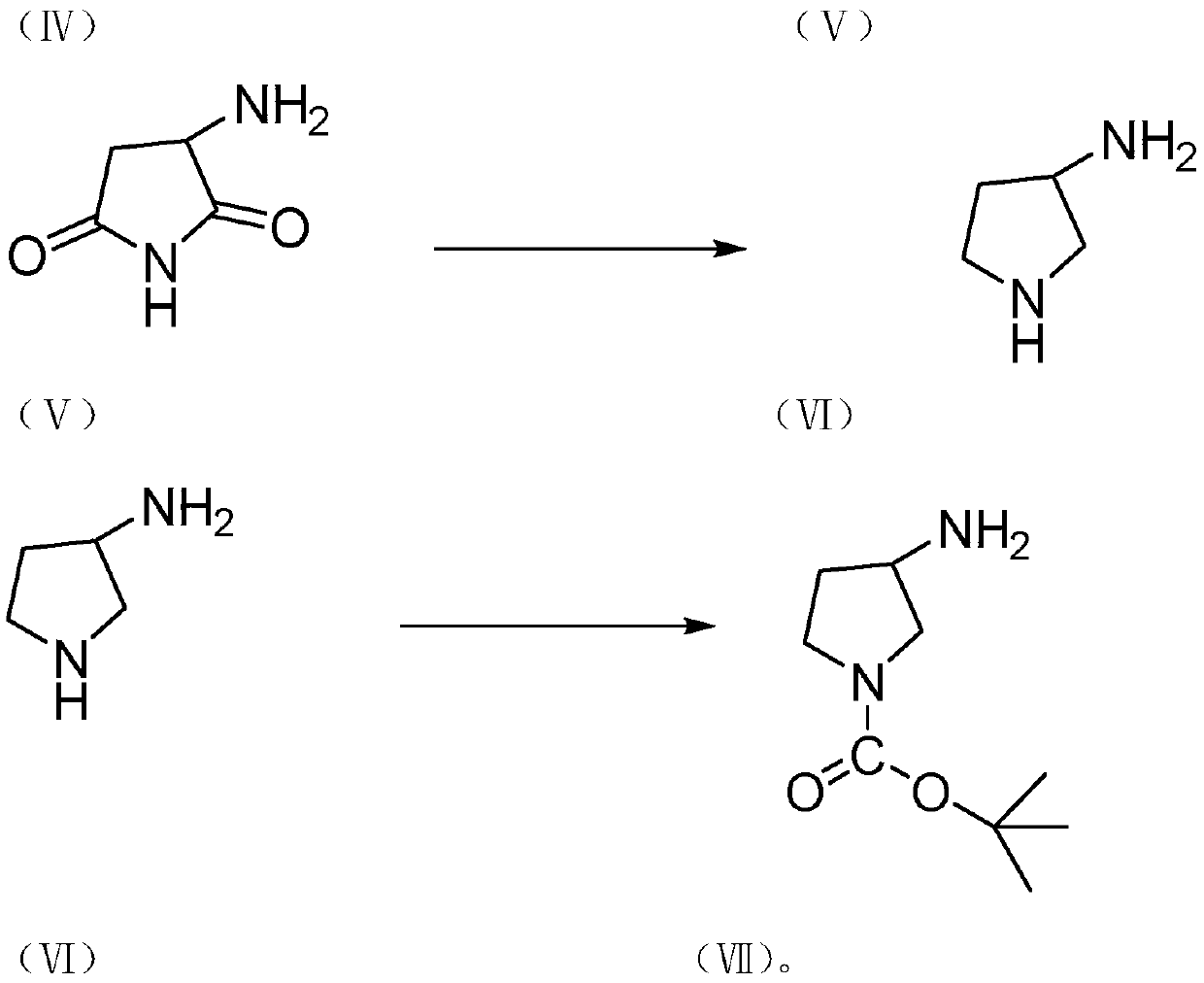
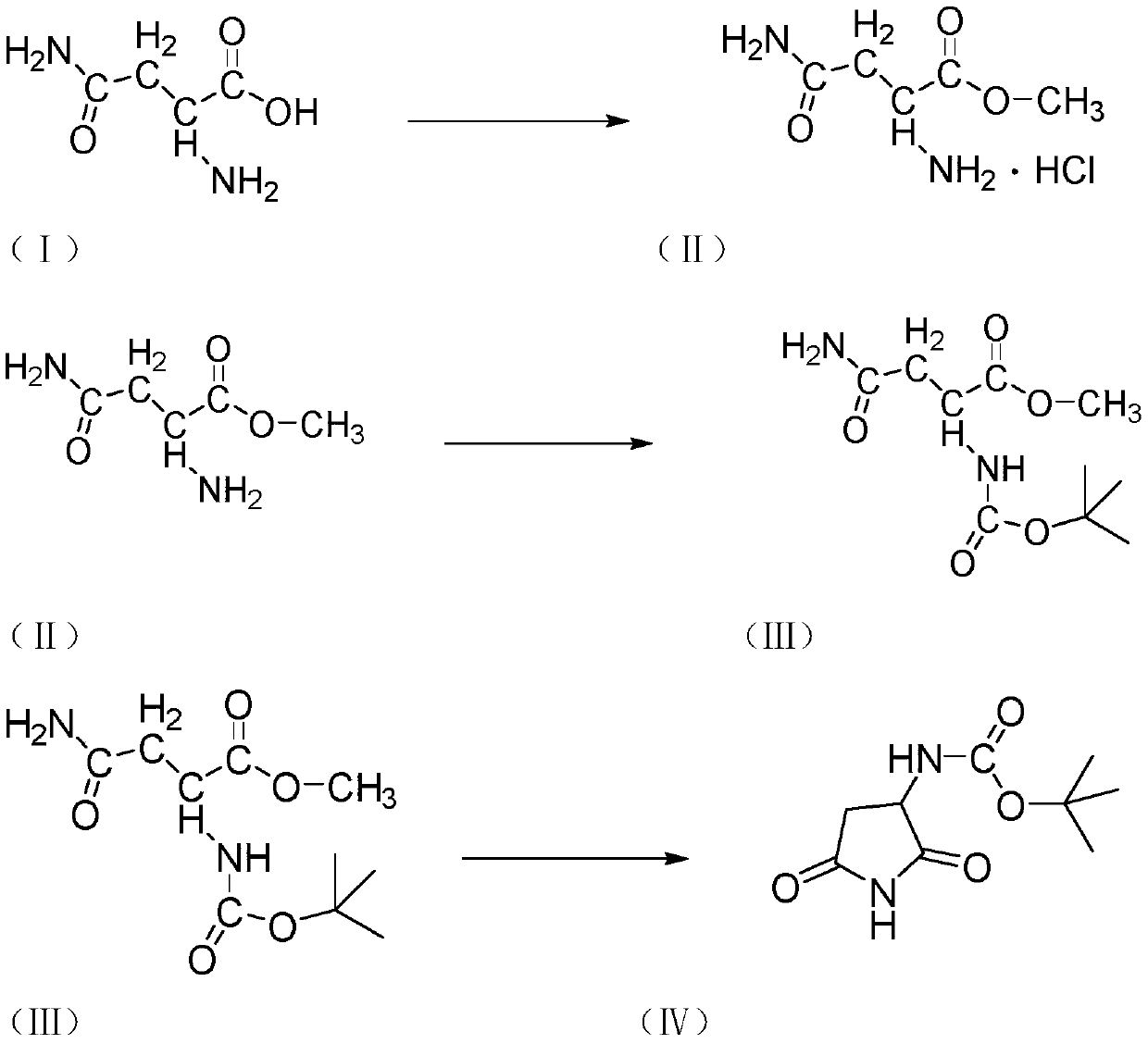
Preparation method of ceftobiprole ester intermediate (R)-1-tert-butyloxycarbonyl-3-aminopyrrolidine
A technology of tert-butoxycarbonyl and aminopyrrolidine, which is applied in the field of preparation of cefepirox intermediates, can solve problems such as being unsuitable for industrialized production, and achieve the effects of being beneficial to industrialized production, low in cost, and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of D-asparagine methyl ester hydrochloride in the step (A)
[0037] Protect the three-necked flask with 2g of methanol and nitrogen gas, cool down to 0°C, slowly drop in 0.27g of thionyl chloride, keep it warm for 1 hour, add 0.5g of D-asparagine, and raise the temperature to 25°C for 5 hours, then complete the reaction.
[0038] Then adjust to acidity with dilute hydrochloric acid, filter, the filter cake is beaten with ether for 1 h, and filter to obtain a white powder with a molar yield of 80%-85%, and LC>95%.
[0039] Preparation of (tert-butoxycarbonyl) asparagine methyl ester in step (B)
[0040] A three-neck flask was passed through a solution of 0.75 g of di-tert-butyl dicarbonate in 7 g of tetrahydrofuran under nitrogen protection, and 0.5 g of D-asparagine methyl ester hydrochloride was added into the flask and suspended in 5 g of tetrahydrofuran and 0.55 g of triethylamine. Cool the resulting white suspension to 0°C, and add di-tert-butyl dic...
Embodiment 2
[0058] The preparation of D-asparagine methyl ester hydrochloride in the step (A)
[0059] Protect the three-necked flask with 2g of methanol and nitrogen gas, cool down to 0°C, slowly drop in 0.29g of thionyl chloride, keep it warm for 1 hour, add 0.5g of D-asparagine, and raise the temperature to 25°C for 5 hours to complete the reaction.
[0060] Then adjust to acidity with dilute hydrochloric acid, filter, the filter cake is beaten with ether for 1 h, and filter to obtain a white powder with a molar yield of 80%-85%, and LC>95%.
[0061] Preparation of (tert-butoxycarbonyl) asparagine methyl ester in step (B)
[0062] A three-neck flask was passed through a solution of 0.78 g of di-tert-butyl dicarbonate in 7 g of tetrahydrofuran under nitrogen protection, and 0.5 g of D-asparagine methyl ester hydrochloride was added into the flask and suspended in 5 g of tetrahydrofuran and 0.55 g of triethylamine. Cool the resulting white suspension to 0°C, and add di-tert-butyl dicarb...
Embodiment 3
[0080] The preparation of D-asparagine methyl ester hydrochloride in the step (A)
[0081] Protect the three-necked flask with 2g of methanol and nitrogen gas, lower the temperature to 0°C, slowly drop in 0.35g of thionyl chloride, keep it warm for 1 hour, add 0.5g of D-asparagine, and raise the temperature to 25°C for 5 hours to complete the reaction.
[0082] Then adjust to acidity with dilute hydrochloric acid, filter, the filter cake is beaten with ether for 1 h, and filter to obtain a white powder with a molar yield of 80%-85%, and LC>95%.
[0083] Preparation of (tert-butoxycarbonyl) asparagine methyl ester in step (B)
[0084] A three-neck flask was protected by nitrogen protection with a solution of 0.66 g of di-tert-butyl dicarbonate in 7 g of tetrahydrofuran, and 0.5 g of D-asparagine methyl ester hydrochloride was added into the flask and suspended in 5 g of tetrahydrofuran and 0.55 g of triethylamine. Cool the resulting white suspension to 0°C, and add di-tert-but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com