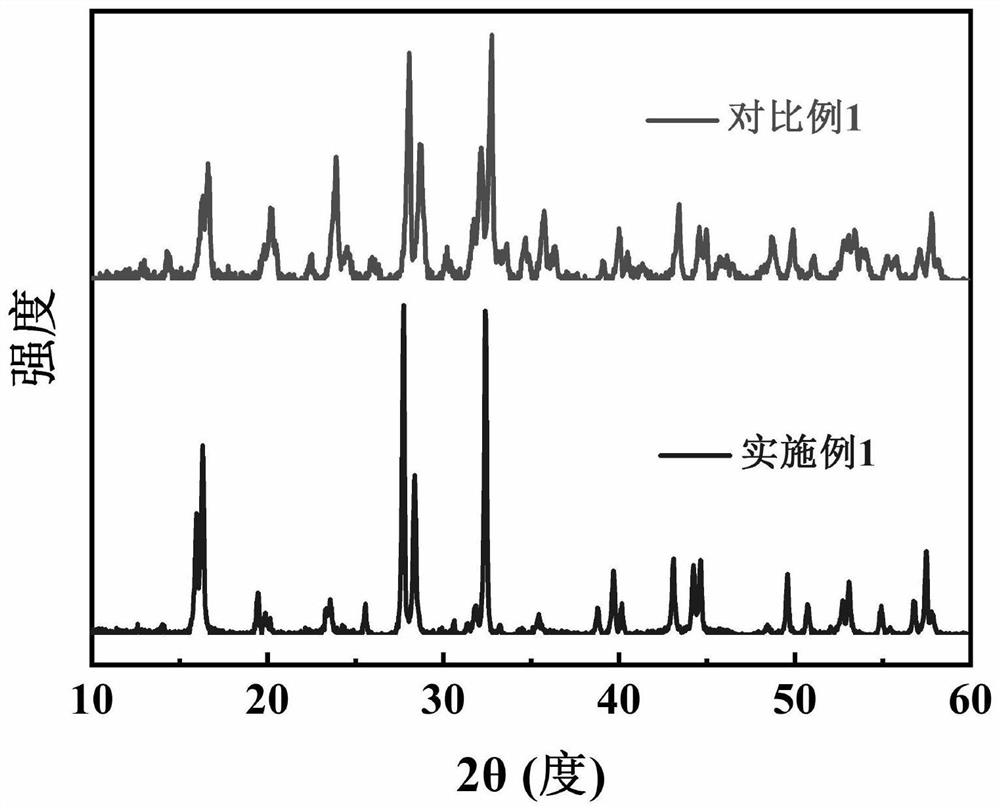
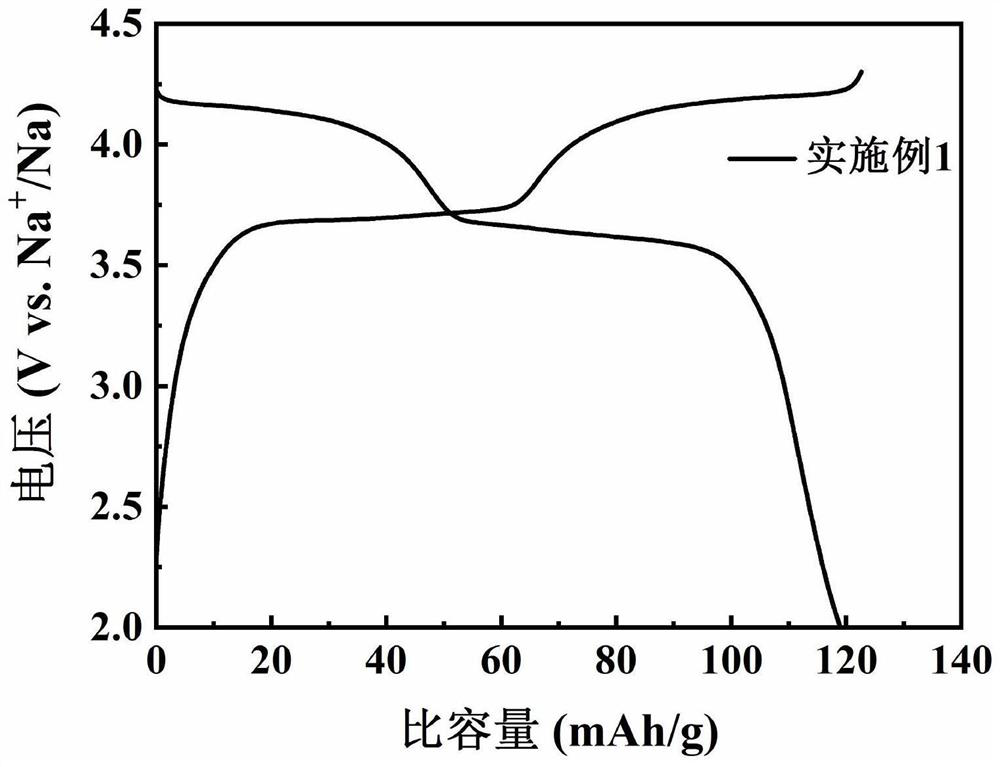
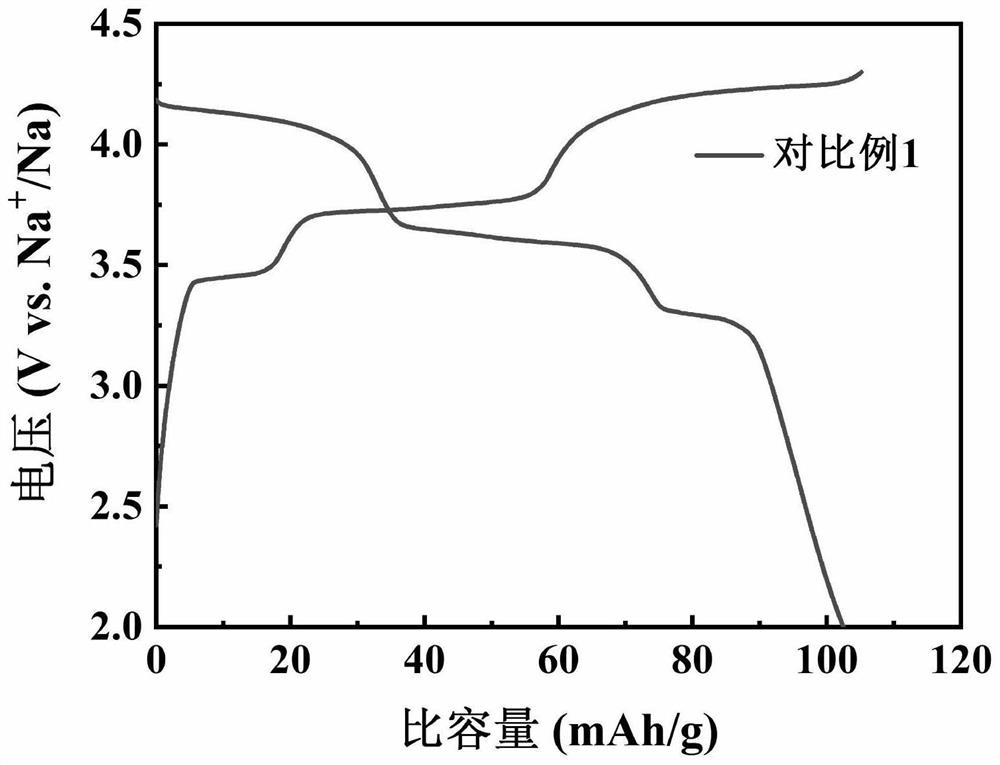
Preparation method of sodium vanadium trifluorophosphate serving as positive electrode material of sodium-ion battery
A technology of sodium vanadium trifluorophosphate and sodium ion batteries, which is applied in the direction of battery electrodes, positive electrodes, secondary batteries, etc., can solve the problems of battery operating voltage and energy density reduction, cycle stability deterioration, etc., to improve the average working Effects of voltage and energy density, energy density value increase, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0030] Specific embodiment one: what this embodiment records is a kind of preparation method of sodium vanadium trifluorophosphate sodium ion battery cathode material, the 3.3V (vs. Na + / Na) left and right discharge voltage platform, described method comprises the steps:
[0031] Step 1: Dissolve the carbon source in deionized water at room temperature, stir and mix evenly to obtain a clear solution M 1 , adjust M 1 The pH is weakly acidic; in this step, the total molar amount of carbon source added is affected by the solution M 1Restriction on the maximum dissolved amount of raw materials, the carbon source added and other raw materials added subsequently (vanadium source, phosphorus source, sodium source and fluorine source) must be completely dissolved in the solution during the operation;
[0032] Step 2: Weigh the vanadium source with a molar ratio of 5:2 to the total carbon source, and add it to the solution M 1 In , a clear solution M was obtained after maintaining...
specific Embodiment approach 2
[0037] Specific embodiment two: the preparation method of a kind of sodium-ion battery cathode material sodium vanadium trifluorophosphate described in specific embodiment one, in step one, described carbon source is organic acid and / or ammonium salt, and described organic acid is Citric acid monohydrate and / or anhydrous citric acid; the ammonium salt is a mixture of one or more of ammonium dihydrogen citrate, diammonium hydrogen citrate, and triammonium citrate. When the first type and the second type of carbon source are selected and added at the same time, the acid and the acidic ammonium salt can form a buffering pH conjugate acid-base pair in the solution.
specific Embodiment approach 3
[0038] Embodiment 3: In the preparation method of sodium vanadium trifluorophosphate, a positive electrode material for a sodium ion battery described in Embodiment 1 or 2, in step 1, the weak acidity means that the pH is 3.0-5.0. The purpose is to accelerate the complexation reaction in step two on the one hand, and to suppress the hydrolysis of fluorine and reduce its volatilization on the other hand.
[0039] Specific embodiment four: a kind of preparation method of sodium vanadium trifluorophosphate sodium ion battery anode material described in specific embodiment three, the mode of adjusting solution to be weakly acidic comprises two kinds: the one, drop ammoniacal liquor directly in the solution; On the premise that the total amount of carbon sources to be added has been selected in step 1, organic acid carbon sources and ammonium salt carbon sources are selected to be added at the same time, and it is realized by adjusting the ratio and corresponding amount of these two...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com