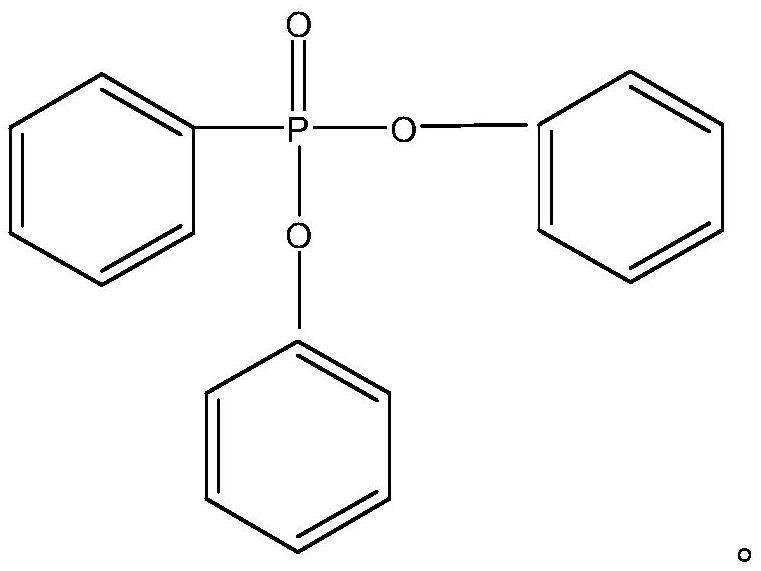
Preparation method of diphenyl phenylphosphonate
A technology of diphenyl phenyl phosphonate and triphenyl phosphite, which is applied in the field of preparation of diphenyl phenyl phosphonate, can solve problems such as difficult industrial production, environmental pollution, and difficult separation, and achieve environmental protection. Less pollution, simple post-treatment, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of preparation method of diphenyl phenylphosphonate, comprising the following steps:
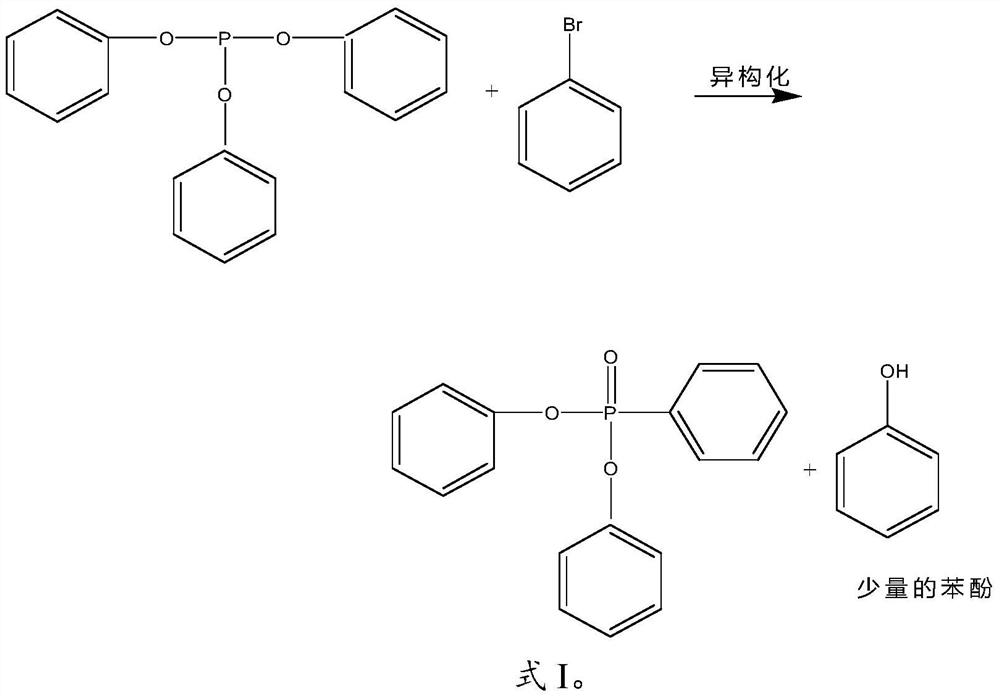
[0030] (1) Under Raney nickel catalysis and protective atmosphere conditions, using benzene bromide as an initiator, triphenyl phosphite is carried out to isomerization reaction, and the gained reaction feed liquid is subjected to vacuum distillation and solid-liquid separation successively to obtain separating liquid;
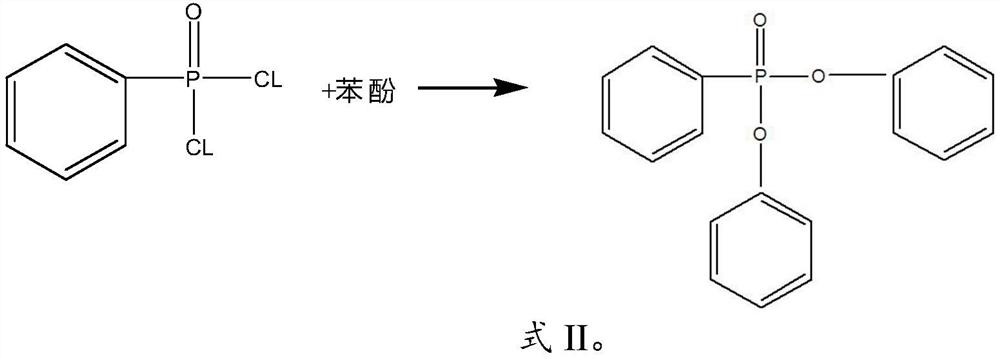
[0031] (2) The separation liquid and phenylphosphonic acid dichloride are mixed for esterification reaction to obtain diphenyl phenylphosphonic acid;
[0032] Wherein, the molar ratio of triphenyl phosphite to benzene bromide is 1:(0.5-2); the temperature of the isomerization reaction is 220-260°C.
[0033] In the invention, under the conditions of Raney nickel catalysis and protective atmosphere, benzene bromide is used as an initiator to perform isomerization reaction on triphenyl phosphite. In the present invention, the protective at...
Embodiment 1
[0046] 1. Wet catalyst Raney nickel (8kg, dosage is 2.58% of the mass of triphenyl phosphite, Raney nickel particle size 50μm) is added to the reaction kettle, and the temperature is raised to 120~ After vacuum dehydration at 125°C for 2 hours, slowly add triphenyl phosphite (310kg, 1kmol) under nitrogen protection, raise the temperature to 240-250°C, start to add benzene bromide (80kg, 0.51kmol) dropwise, and add dropwise for 2h , keep warm for half an hour after the dropwise addition, monitor by liquid chromatography, and stop the reaction after monitoring the complete reaction of the raw material triphenyl phosphite;
[0047] The monitoring data in the reaction process are shown in Table 1:
[0048] Monitoring data in the reaction process of table 1
[0049]
[0050] 2. Lower the temperature of the reaction feed solution in step 1 to 70-80°C, recover excess benzene bromide under reduced pressure, the vacuum degree is 2-3mmHg, the recovery temperature is 130-135°C, and a...
Embodiment 2
[0058] 1. Catalyst Raney nickel (7 kg of recovered Raney nickel, add 1 kg of dried new catalyst, the dosage is 2.58% of the mass of triphenyl phosphite, and the Raney nickel particle size is 50 μm) into the reaction kettle, add phosphorous acid Triphenyl ester (310kg, 1kmol), under the protection of nitrogen, the temperature was raised to 240-250°C, and benzene bromide (80kg, 1.02kmol) was added dropwise for 3 hours. The method is monitored, and the reaction is stopped after monitoring the complete reaction of the raw material triphenyl phosphite;
[0059] The monitoring data during the reaction are shown in Table 2:
[0060] Table 2 reaction process monitoring data
[0061]
[0062]
[0063] 2. After the reaction, cool down to 70-80°C, and start to reduce the pressure to recover excess benzene bromide. The vacuum degree is 2-3mmHg, and the recovery temperature is 140-145°C. After recovery, filter the catalyst under nitrogen pressure filtration at 60-65°C , and the cat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com