Preparation method and application of 2, 4-diamino-6-hydroxy-5-formamidopyrimidine
A technology of hydroxypyrimidine and diamino, which is applied in the field of preparation of intermediates of Lowe antiviral drugs, can solve the problems of high raw material cost, low safety, and large amount of "three wastes", and achieve high atom utilization, yield and The effect of high purity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Dissolve 50g of nitrosopyrimidine (MW155.11, 0.32mol) and 5g of sodium metabisulfite (MW190.11, 0.03mol) in 50g of formamide (MW45.04, 1.11mol) and 50g of water (MW18.02, 2.78mol) In the process, slowly raise the temperature to 70-80°C, keep the temperature for 2 hours, then raise the temperature to 105-115°C, and keep the temperature for 3 hours.
[0061] At the same time, the ammonia and carbon dioxide generated during the reaction were passed into 100 g of ice water to prepare a 33% aqueous solution of ammonium carbonate (which can be used for the free purification of guanine).
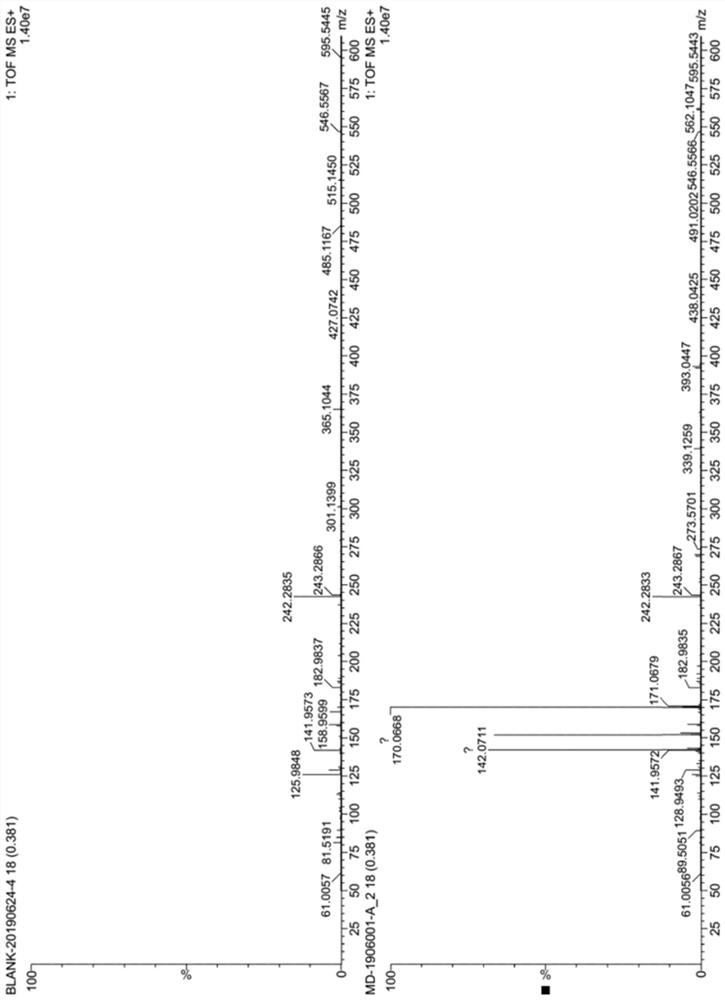
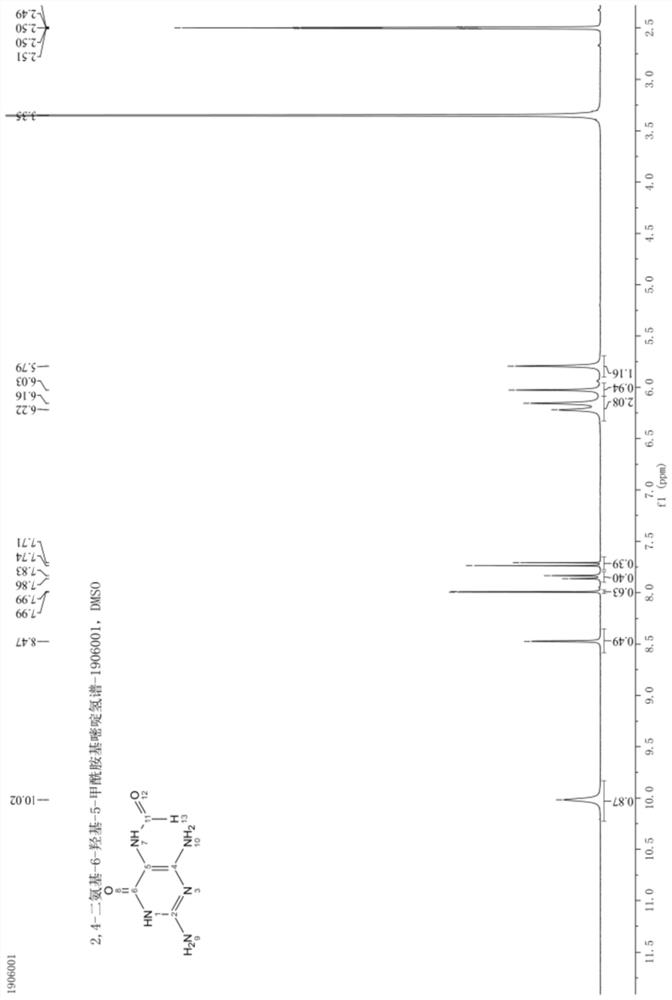
[0062] After the reaction is complete, add 150g of water, stir to dissolve, cool down to 5-15°C, keep the crystal for 2 hours, filter, and dry to obtain 53.2g of off-white solid powder, which is the target product 2,4-diamino-6- Hydroxy-5-carboxamidopyrimidine, the molar yield is 97.0%, and the purity is 99.0%.
[0063] The structural analysis data of the 2,4-diamino 6-hydroxyl-5-carboxamid...
Embodiment 2
[0067] Dissolve 200g of nitrosopyrimidine (MW155.11, 1.29mol) and 30g of sodium metabisulfite (MW190.11, 0.16mol) in 180g of formamide (MW45.04, 4.00mol) and 250g of water (MW18.02, 13.87mol) In the process, slowly raise the temperature to 70-80°C, keep the temperature for 2 hours, then raise the temperature to 105-115°C, and keep the temperature for 3 hours.
[0068] At the same time, the ammonia and carbon dioxide generated during the reaction were introduced into 400 g of ice water to prepare a 33% ammonium carbonate aqueous solution (which can be used for the free purification of guanine).
[0069] After the reaction is complete, add 600g of water, stir to dissolve, cool down to 5-15°C, keep the crystal for 2 hours, filter, and dry to obtain 213.0g of off-white solid powder, which is the target product 2,4-diamino-6- Hydroxy-5-carboxamidopyrimidine, the molar yield is 97.0%, and the purity is 99.0%.
Embodiment 3
[0074] Dissolve 50g (MW169.14, 0.29mol) of 2,4-diamino-6-hydroxy-5-carboxamidopyrimidine in 150g of 85% formic acid (MW46.03, 2.77mol), heat up to 120-130°C and reflux React for 13 hours.
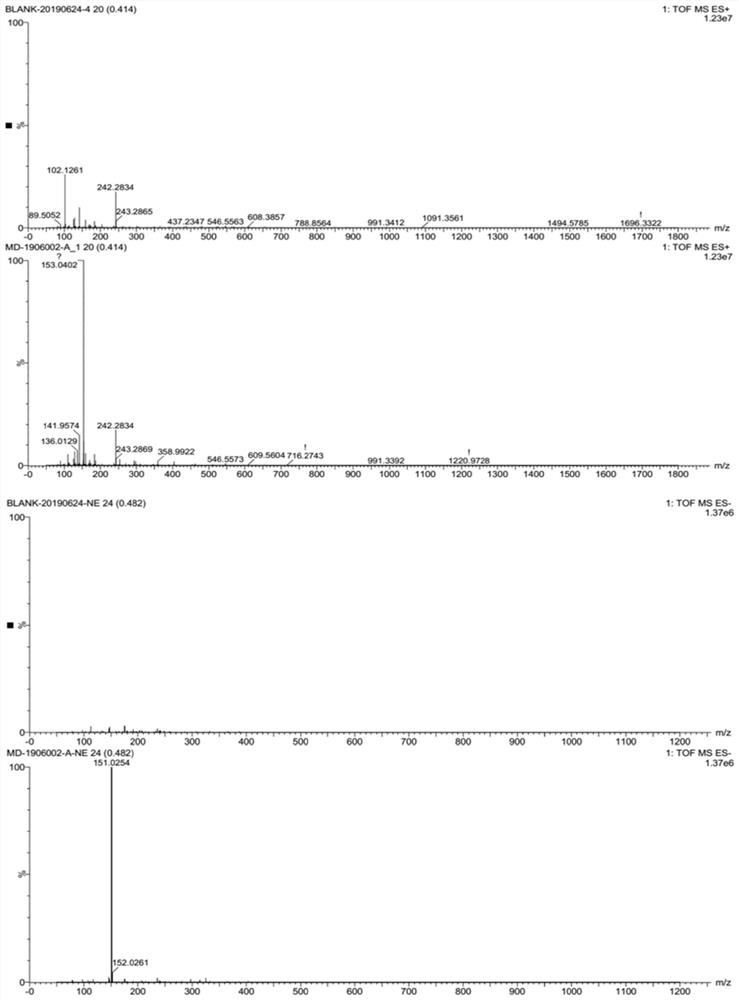
[0075] After the reaction is complete, formic acid is reclaimed by distillation (recovering formic acid can be applied mechanically), 100 g of water is added, and after being cooled to room temperature, it is stirred and beaten for 30 minutes, and filtered to obtain 85 g of crude guanine. Dissolve the crude guanine formate in 200g of water, raise the temperature to 70-80°C, add dropwise 33% ammonium carbonate aqueous solution, adjust the pH of the reaction system to 7.5-8.5, stir for 0.5 hours, filter and dry to obtain 42.5g off-white The solid powder is the finished product of guanine, with a molar yield of 95% and a purity of 99.0%.
[0076] Its structural analysis data of the guanine prepared in the present embodiment are as follows:
[0077] ESI-MS(M / Z): 151.0[M] or 153.0[M+2H] such a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com