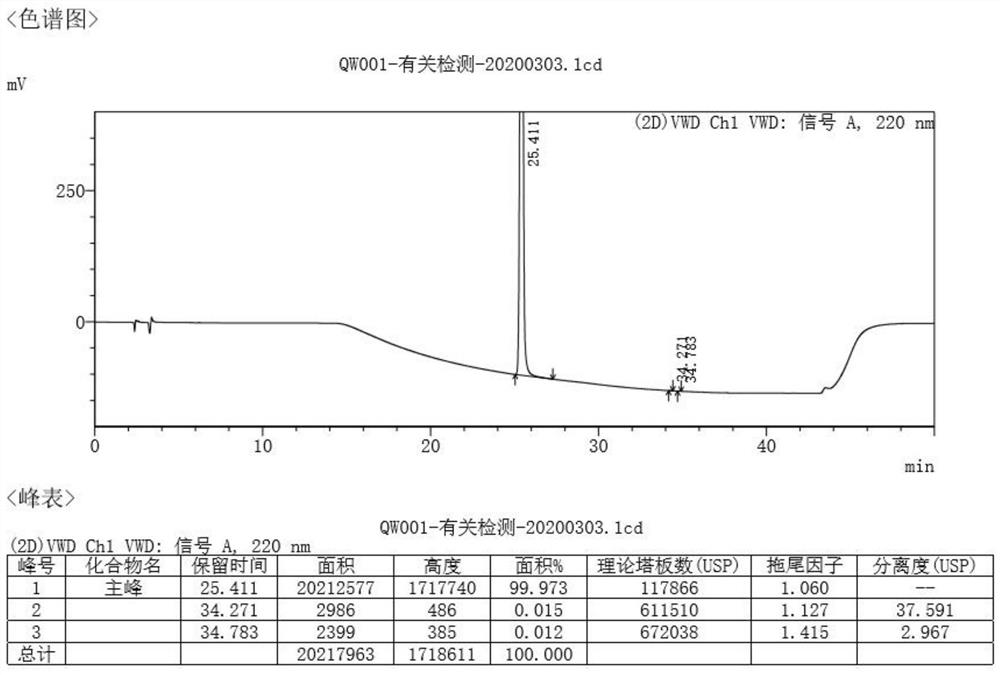
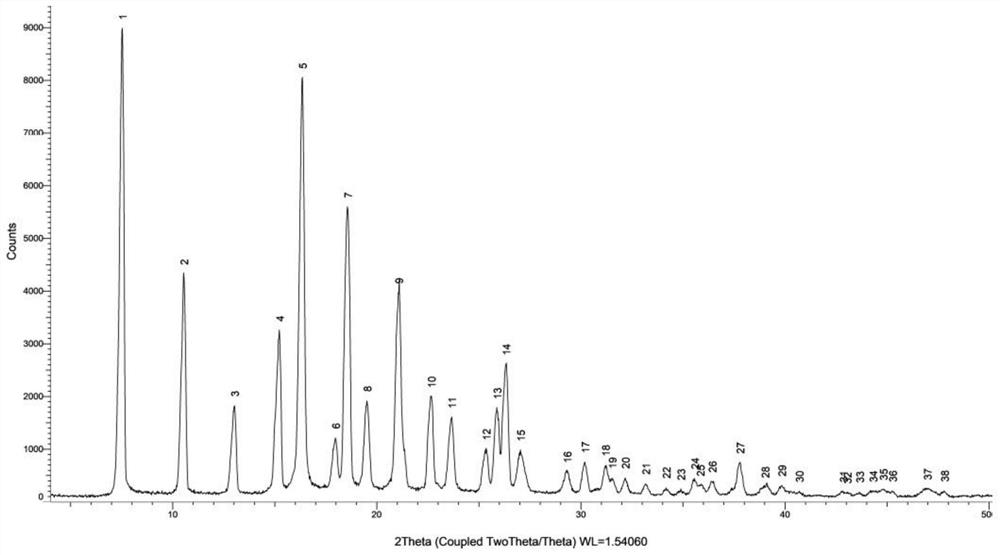
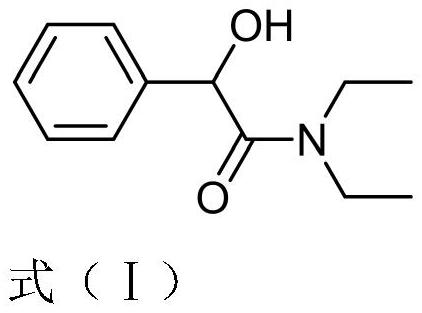
Crystal form of N,N-diethyl-2-hydroxy-2-phenylacetamide as well as preparation method and application of crystal form
A technology of phenylacetamide and diethyl, which is applied in the preparation of carboxylic acid amide, botany equipment and method, and the preparation of organic compounds, etc., can solve the problems of economy and environmental protection that are not suitable for large-scale production, and achieve the goal of being suitable for carrying and Transport, reduce production costs, and reduce the effects of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] A preparation method of N,N-diethyl-2-hydroxyl-2-phenylacetamide crystal form, using mandelic acid as a raw material, condensation reaction with cyclohexanone, and then aminolysis reaction with diethylamine, Obtaining the crystal form of N,N-diethyl-2-hydroxyl-2-phenylacetamide specifically comprises the following steps:
[0035] (1) Dissolve mandelic acid [formula (II)] in solvent A, and react with cyclohexanone at 20°C to 200°C for 1h to 96h. After the reaction is complete, filter and concentrate to obtain crude product A [formula (III) ]; Wherein the molar ratio of mandelic acid, solvent A and cyclohexanone consumption is 1: (1~8): (1~8);
[0036]
[0037] (2) Add solvent B to the crude product A, stir, crystallize and centrifuge to obtain the pure product A, heat the pure product A and diethylamine at 20°C to 100°C for 1h to 144h, after the reaction is complete, filter Concentrate, wash and dry to obtain the crude product B [formula (I)]; wherein the molar ratio...
Embodiment 1
[0045] Example 1 Preparation of N,N-diethyl-2-hydroxyl-2-phenylacetamide crystal form 1
[0046] At room temperature, add 15kg of formula (II) to a 50L reaction kettle, inhale 18.2kg of toluene and 14.5kg of cyclohexanone under negative pressure, keep the reaction at 150°C for 48 hours, and separate water during the reaction. After the reaction is complete, the reaction is processed. After the reaction solution is hot-filtered to remove insoluble matter, it is concentrated to remove the solvent to obtain the crude product of formula (Ⅲ). Add 50L of n-hexane to stir and crystallize. After 5 hours, a solid is precipitated, and it is centrifuged to dry to obtain the pure product of formula (Ⅲ). 22.2kg with a purity of 98.0%.
[0047] At room temperature, add the above-mentioned pure product of formula (III) and 21 kg of diethylamine to a 50 L reaction kettle respectively, heat at 50° C. for 72 hours, and perform post-treatment after the reaction is complete. After the reaction s...
Embodiment 2
[0049] Example 2 Preparation of N,N-diethyl-2-hydroxyl-2-phenylacetamide crystal form 2
[0050] At room temperature, add 15kg of formula (II) to a 50L reaction kettle, inhale 31.4kg of xylene and 9.7kg of cyclohexanone under negative pressure, keep the temperature at 110°C for 60 hours, and separate water during the reaction. After the reaction was complete, the reaction was processed, and the solvent was concentrated to remove the crude product of formula (Ⅲ). Add 40 L of petroleum ether to stir and crystallize. After 5 hours, a solid precipitated, and was centrifuged to dry to obtain 21 kg of pure product of formula (Ⅲ), with a purity of 97.1%.
[0051] At room temperature, add the above-mentioned pure product of formula (III) and 7.3 kg of diethylamine to a 50 L reaction kettle respectively, and react at 70° C. for 72 hours. After the reaction is complete, the reaction is post-processed. The reaction solution was concentrated to remove the solvent to obtain a residue. To t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com