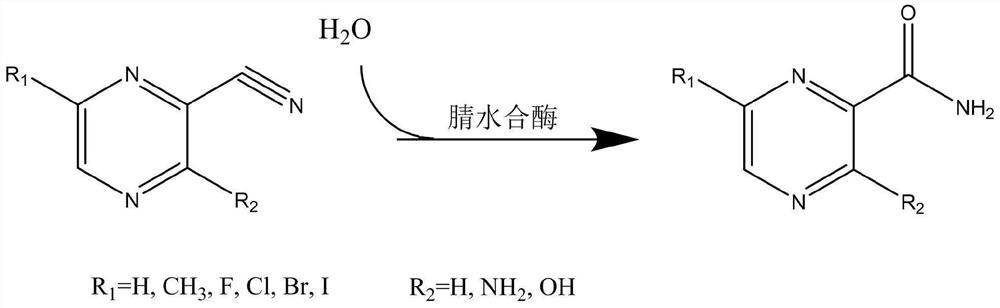
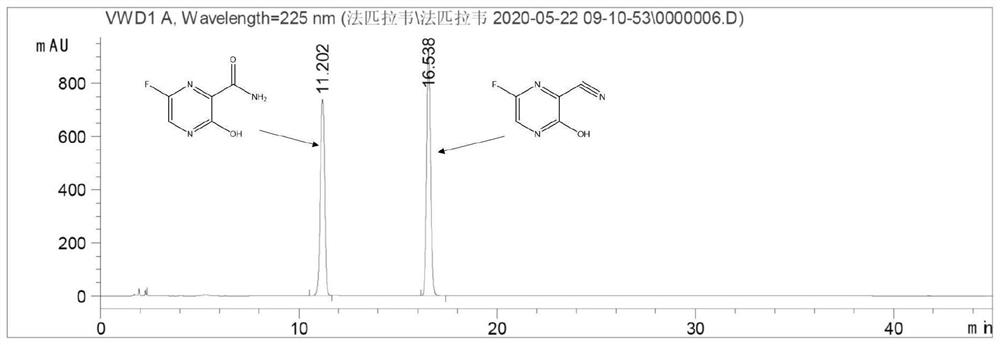
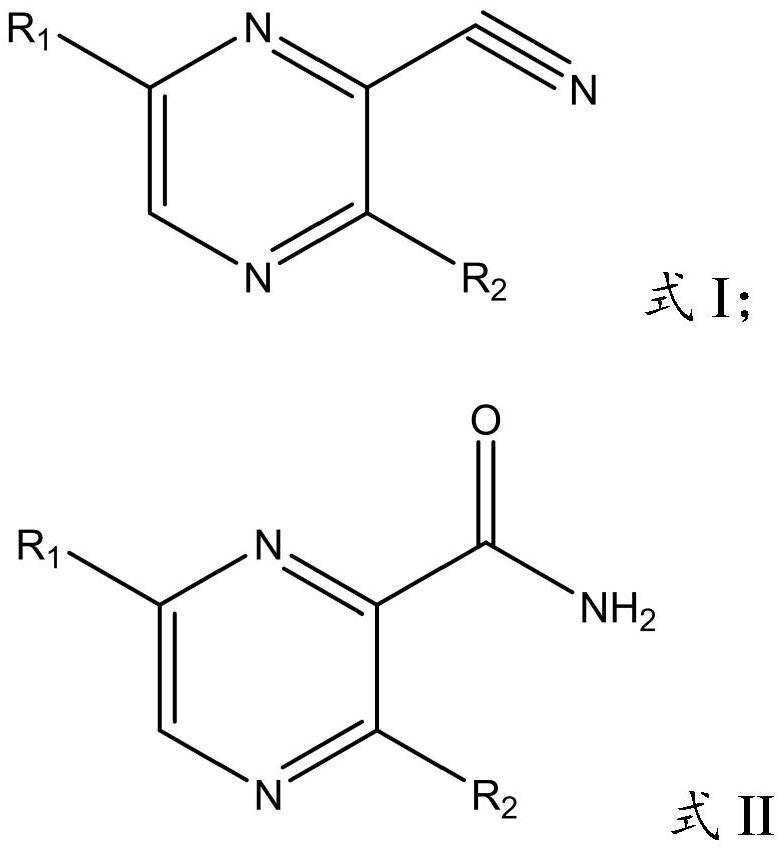
Application of nitrile hydratase in catalyzing hydration reaction of cyanopyrazine compound to generate amide pyrazine compound
A technology for catalyzing cyanopyrazine and nitrile hydratase, which is applied in the direction of enzymes, lyases, and carbon-oxygen lyases, can solve the problems of complex synthesis, low conversion rate and total yield, cumbersome process, etc., and achieve high conversion rate , no side effects, the effect of green environmental protection operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] In the present invention, the preparation method of the cell (genetically engineered bacterium) expressing nitrile hydratase in vitro comprises: constructing the gene expressing nitrile hydratase into a target plasmid vector, introducing it into the expression host bacterium, and obtaining the cell (genetic engineering bacterium) expressing nitrile hydratase in vitro cells; the target plasmid vector is preferably pET-30a(+), pET-21a(+), pET-22b(+), pET-28a(+), pETDuet-1 or pACYCDuet-1, but not limited to the These vectors are preferably pET-28a(+), and the enzyme cutting sites are preferably EcoRI / BamHI and HindIII; the expression host bacteria are preferably Escherichia coli, more preferably E.coliBL21(DE3). The present invention has no special limitation on the specific method for constructing the gene expressing nitrile hydratase into the target plasmid vector and introducing it into the expression host bacterium, and conventional methods in the art can be used.
[0...
Embodiment 1
[0063] 1. Acquisition of nitrile hydratase gene
[0064] The amino acid sequences of the reported cobalt-type nitrile hydratases were collected, and the ClustalW2 method (http: / / www.ebi.ac.uk / Tools / msa / clustalw2) was used to perform multiple sequence alignments on the nitrile hydratases to determine the the conservative region. The amino acid sequence of the conserved region was used as a molecular probe for the subsequent search of the nitrile hydratase gene in the gene information database. By comparing the reported amino acid sequences of nitrile hydratase, a conservative amino acid sequence in its α subunit -CTLCSC- was selected as a molecular probe, using BasicLocalAlignmentSearchTool (http: / / blast.ncbi.nlm.nih.gov) Homologous sequences were searched in the GenBank bioinformatics database (https: / / www.ncbi.nlm.nih.gov / genbank / ). Genes that have been reported and annotated as nitrile hydratase after whole-genome sequencing were selected. The amino acid sequences of the ...
Embodiment 2
[0083] Example 2 Genetically engineered bacteria catalyze 2-cyanopyrazine to generate 2-amide pyrazine
[0084] Get the fermented liquid of the engineered bacterium E.coliBL21(DE3) / pET-28a(+)-NH16 that 25ml embodiment 1 constructs, 12000rpm, 10min centrifugal collection thalline, then reconstitute with the buffer solution of 250ml50mM Tris-HCl (pH8.0) Suspend the collected bacteria, and the enzyme activity of the resuspended enzyme solution is 10U / ml. Add 1.0 g of 2-cyanopyrazine to the resuspension, and carry out hydration reaction at 20°C for 24 hours. Then liquid chromatography was used to detect the content of 2-cyanopyrazine, 2-amide pyrazine and 2-carboxypyrazine in the reaction system. The conversion rate of the substrate is greater than 99%, the yield of 2-amide pyrazine is greater than 92%, and no generation of 2-carboxypyrazine is found in the reaction system.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com