Homogeneous chemiluminiscence detection method and application thereof
A homogeneous chemiluminescence, detection method technology, applied in chemical instruments and methods, chemiluminescence/bioluminescence, analysis by chemical reaction of materials, etc. Label signal detection and other problems, to achieve the effect of eliminating matrix effects, low cost, and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1: Preparation of a chemiluminescent microarray chip for simultaneous detection of cTnI, CK-MB, MYO, and NT-proBNP.
[0146] 1. Modification of the surface of the solid phase support.
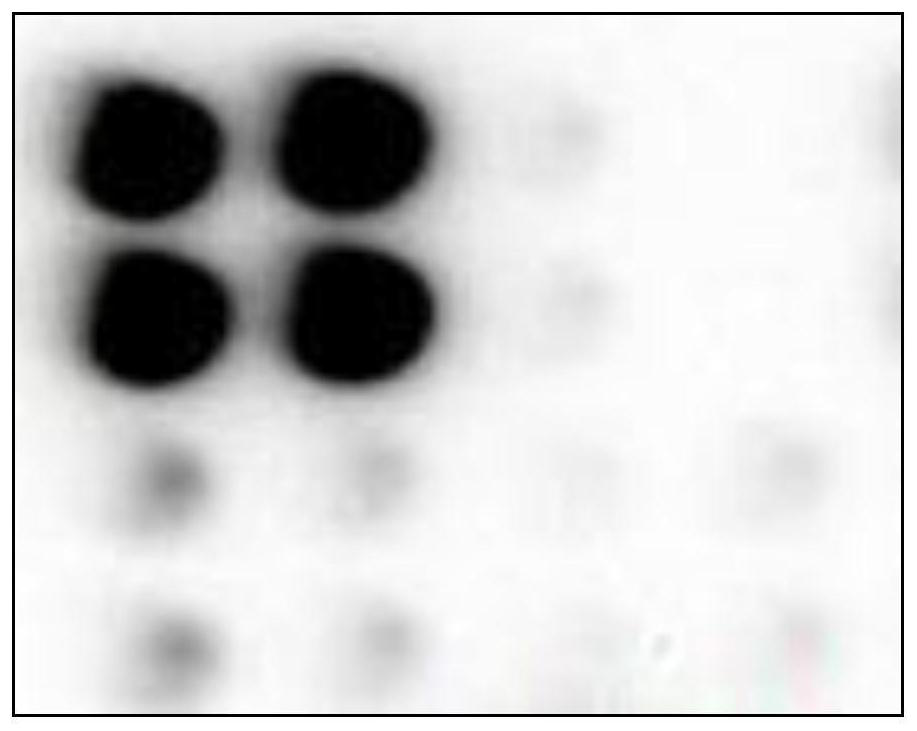
[0147] On the glass chip with 4×4 layout, there are 16 reaction areas, and the surface of each reaction area is modified with groups that can be connected with biotin molecules.
[0148] (1) Prepare 20mg / mL biotin solution with DMSO,
[0149] (2) with 0.1M NaHCO 3 Solution Dilute the biotin solution to 1 mg / ml.
[0150] (3) Soak the modified glass chip in the above biotin solution, and let it stand at 2-8° C. for 12-16 hours to react.
[0151] (4) Take out the glass chip and use 0.1M NaHCO 3 The solution was washed to obtain a solid phase support (glass chip) whose surface was modified with biotin.
[0152] 2. Preparation of donor microspheres coated with avidin
[0153] 1) Preparation of donor microspheres
[0154] (1) Prepare a 25 mL round bottom flask, add 0.1 g of copp...
Embodiment 2
[0180] Example 2: Preparation of receptor microspheres coated with antibody II
[0181] 1) Preparation of acceptor microspheres
[0182] (1) Prepare a 25mL round-bottomed flask, add 0.1g of europium(III) complex, 10mL of 95% ethanol, stir magnetically, and raise the temperature of the water bath to 70°C to obtain a solution of europium(III) complex.
[0183] (2) Prepare a 100mL three-necked flask, add 10mL of 95% ethanol, 10mL of water and 10mL of polystyrene microspheres coated with carboxydextran hydrogel with a particle size of 10% and a particle size of 200nm, stir magnetically, and heat up the water bath to 70°C.
[0184] (3) Slowly add the europium(III) complex solution in step 1 into the three-necked flask in step 2, react at 70°C for 2 hours, stop stirring, and cool naturally to obtain an emulsion.
[0185] (4) Centrifuge the above emulsion for 1 hour at 30,000 g, discard the supernatant after centrifugation, and then resuspend with 50% ethanol. After repeated centr...
Embodiment 3
[0192] Example 3: Using the method of the present invention to simultaneously detect cTnI, CK-MB, MYO, and NT-proBNP in the analytes.
[0193] (1) Take 15ul of the 4 samples and spot them on columns 1, 2, 3, and 4 shown in Table 1.
[0194] (2) Dilute the receptor microspheres coated with cTnI antibody Ⅱ, CK-MB antibody Ⅱ, MYO antibody Ⅱ, and NT-proBNP antibody Ⅱ to 50ug / ml with buffer, respectively, and take 15ul samples and place them in Table 1. Incubate for 10 minutes at 37°C for rows A, B, C, and D shown.
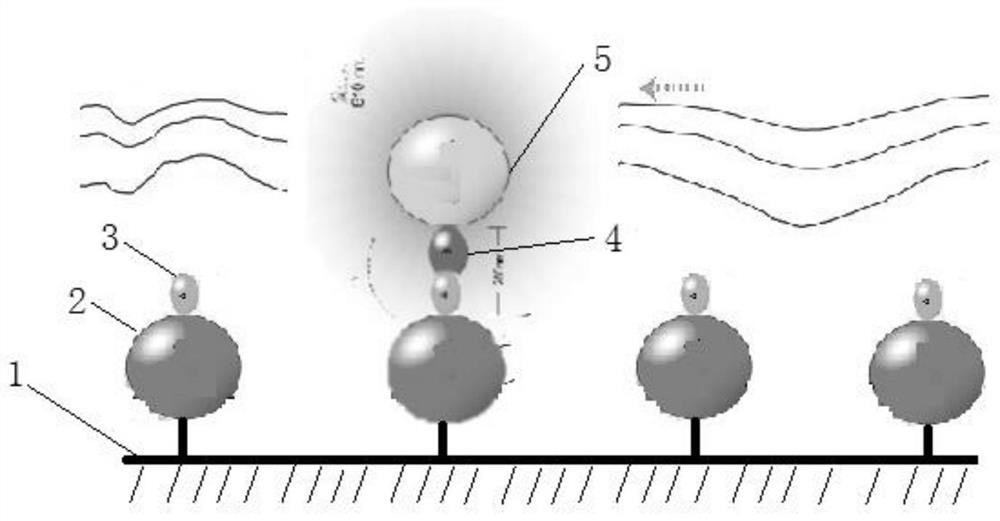
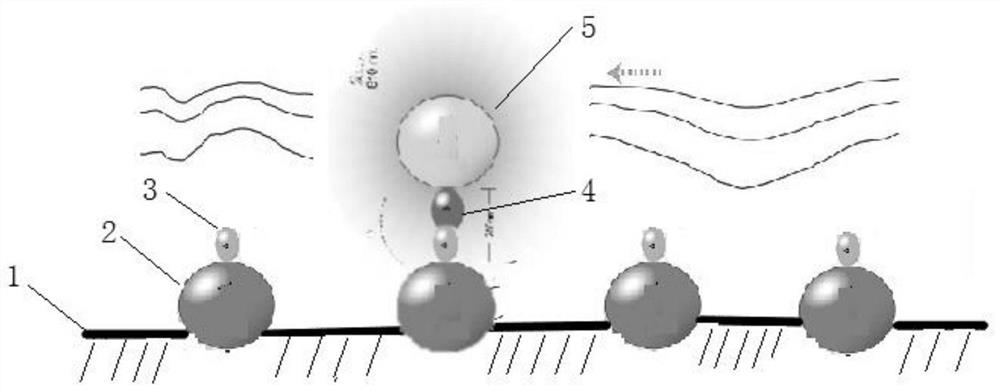
[0195] (3) The glass chip is irradiated with excitation light with a wavelength of 680nm, and the donor microspheres are induced to activate and release active oxygen in a high-energy state. The active oxygen in the high-energy state is captured by the acceptor microspheres at a short distance, thereby transferring energy to activate the luminescent compound in the acceptor microspheres. After a few microseconds, the luminescent compound in the acceptor microsphere w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com