Organic electroluminescent compound, and preparation method and application thereof
A technology of electroluminescent devices and compounds, applied in organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve the problems of quantum efficiency and service life, and achieve stable three-dimensional space, high quantum efficiency, and mild reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
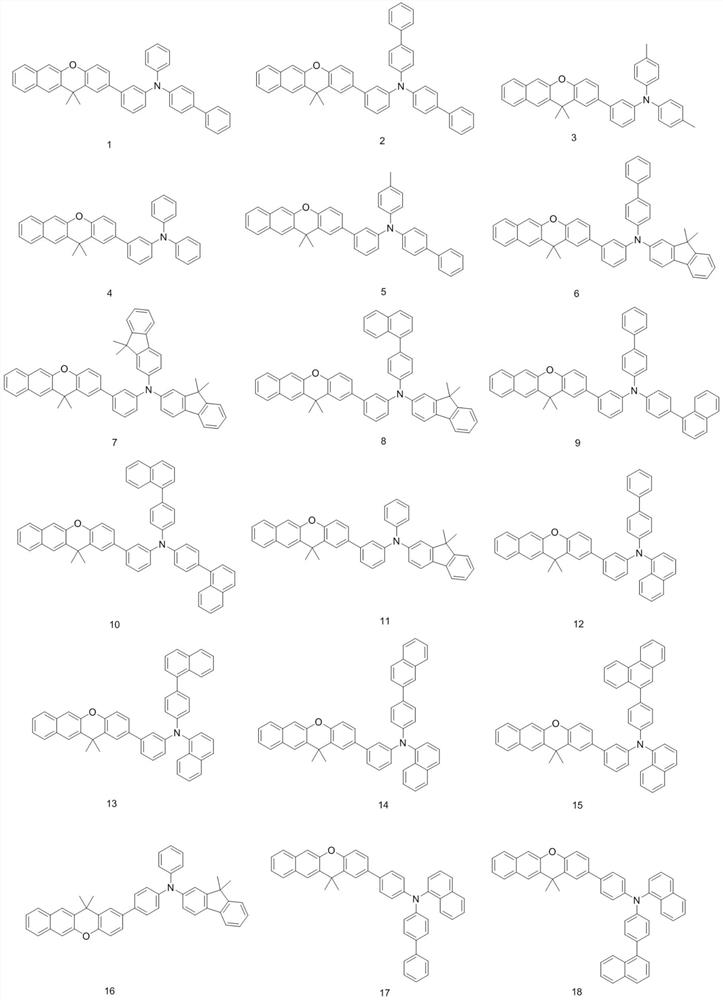
[0056] Embodiment 1: preparation compound 1
[0057]
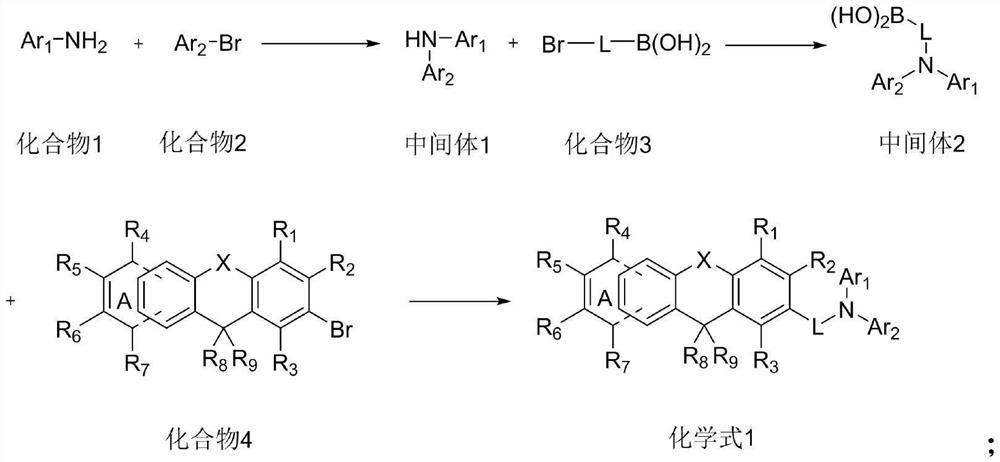
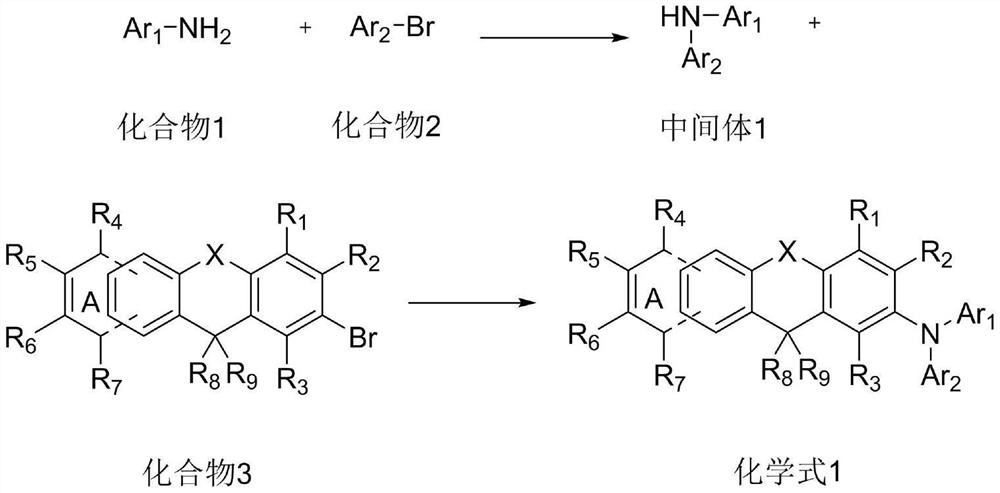
[0058] (1) Add compound 1-1 (210mmol) and compound 2-1 (210mmol) in the reaction vessel, add 300ml of toluene solution and place it in the reaction vessel, replace the gas in the reaction vessel with nitrogen for 3 times, under nitrogen atmosphere Add tris(dibenzylideneacetone)dipalladium (2.1mmol), tri-tert-butylphosphine (10.5mmol) and sodium tert-butoxide (420mmol), heat up to reflux, stir at 80r / min for 5h, the reaction is complete, the reaction is over Afterwards, after the reaction solution was cooled to room temperature, ammonium chloride aqueous solution was added to the reaction solution to complete the reaction, the molar ratio of compound 1 to ammonium chloride was 1:10, and the reaction solution was extracted with ethyl acetate, and then magnesium sulfate was used to The extracted organic layer was dried, and the solvent was removed by heating with a rotary evaporator at 70°C in a water bath, and the remaini...
Embodiment 2
[0061] Embodiment 2: preparation compound 27
[0062]
[0063] (1) Add compound 1-27 (210mmol) and compound 2-27 (210mmol) in the reaction vessel, add 300ml of toluene solution and place it in the reaction vessel, replace the gas in the reaction vessel with nitrogen for 3 times, under nitrogen atmosphere Add tris(dibenzylideneacetone)dipalladium (2.1 mmol), tri-tert-butylphosphine (10.5 mmol) and sodium tert-butoxide (420 mmol), heat up to reflux, stir at 80 r / min for 5 h, and the reaction is complete. After the reaction, the reaction solution was cooled to room temperature, ammonium chloride aqueous solution was added to the reaction solution to complete the reaction, the molar ratio of compound 1 to ammonium chloride was 1:10, and the reaction solution was extracted with ethyl acetate. The extracted organic layer was dried using magnesium sulfate, and the solvent was removed by heating in a 70° C. water bath using a rotary evaporator. The remaining material was purified ...
Embodiment 3
[0065] Embodiment 3: preparation compound 41
[0066]
[0067] (1) Add compound 1-41 (210mmol) and compound 2-41 (210mmol) to the reaction vessel, add 300ml of toluene solution and place it in the reaction vessel, replace the gas in the reaction vessel with nitrogen for 3 times, and add Tris(dibenzylideneacetone)dipalladium (2.1mmol), tri-tert-butylphosphine (10.5mmol) and sodium tert-butoxide (420mmol) were heated to reflux, stirred at 80r / min for 5h, and the reaction was complete. After the reaction, the reaction solution was cooled to room temperature, ammonium chloride aqueous solution was added to the reaction solution to complete the reaction, the molar ratio of compound 1 to ammonium chloride was 1:10, and the reaction solution was extracted with ethyl acetate. The extracted organic layer was dried using magnesium sulfate, and the solvent was removed by heating in a 70° C. water bath using a rotary evaporator. The remaining material was purified by column chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com