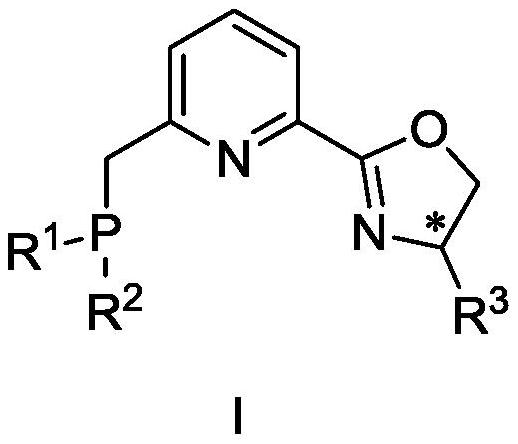
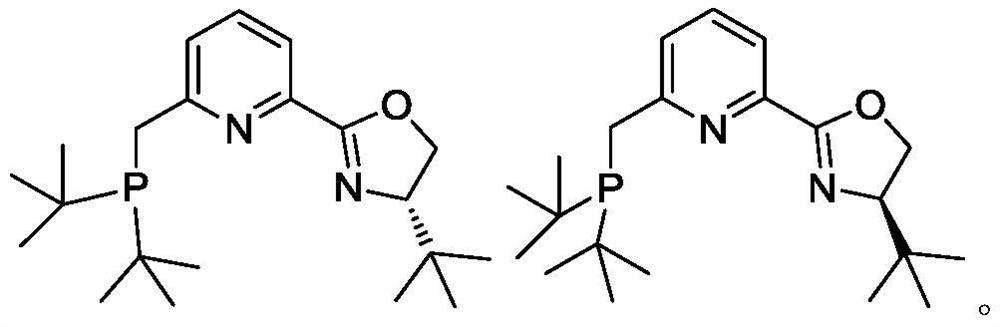
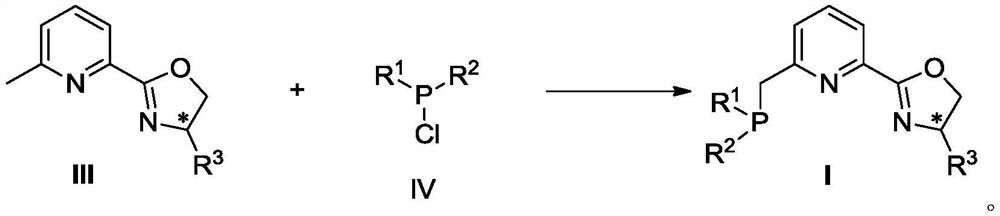
Phosphine pyridine oxazoline compound, metal complex, and preparation method and application thereof
A technology of phosphine oxazoline and metal complex, which is applied in the field of phosphine oxazoline compounds, can solve the problems of low activity and limited asymmetric hydrogenation reaction, and achieves high yield, excellent catalytic activity and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Embodiment 1: Preparation of phosphine pyridine oxazoline compound
[0142]
[0143] Compound IIIa: Under argon protection, add 2-cyano-6-methylpyridine (1.33g, 11.2mmol), Zn(OTf) to a 100mL dry three-necked flask in sequence 2(205mg, 0.56mmol), anhydrous toluene (30mL), stirred for about 10min, then added compound VIa L-tert-leucinol (1.45g, 12.4mmol) in anhydrous toluene (10mL) solution, and the reaction system was heated Reflux for 24h, TLC tracking reaction until complete. The system was cooled to room temperature, diluted with ethyl acetate (30 mL), and then successively washed with saturated NaHCO 3 solution (3×15mL) and saturated NaCl (30mL), washed with anhydrous NaCl 2 SO 4 It was dried, filtered, concentrated, and purified by column chromatography (ethyl acetate:petroleum ether=1:5→1:3) to obtain compound IIIa (1.34 g, 55%) as a white solid.
[0144] Proton spectrum purity>97%. 1 H NMR (400MHz, CDCl 3 )δ7.93(d, J=7.8Hz, 1H), 7.63(t, J=7.7Hz, 1H), 7.23...
Embodiment 2
[0156] Embodiment 2: preparation cobalt complex
[0157]
[0158] Cobalt complex IIa:N 2 In the glove box, weigh anhydrous CoCl 2 (107mg, 0.83mmol) solid in a 50mL Schlenk bottle, add tetrahydrofuran 30mL, stir for 2h until it becomes a homogeneous blue solution, then slowly add compound Ia (300mg, 0.83mmol) to it, the color of the reaction solution gradually changes to Blue ink. After the reaction was stirred at room temperature for 10 h, the mixture was concentrated with an oil pump to obtain a solid, which was washed with an appropriate amount of ether, filtered, and dried in vacuo to obtain compound IIa (351 mg, 94%) as a blue-ink solid.
[0159] Proton spectrum purity>97%.
[0160] 1 H NMR (400MHz, CDCl 3 )δ91.83,69.13,62.45,21.43,19.61,7.17,5.75,1.56,1.54,-0.27,-13.02,-15.19,-15.63,-23.25.
[0161] IR(neat)ν max (cm -1 )=2946,2866,1654,1590,1445,1367,1181,973,932,841,754.
[0162] Elemental analysis (C 21 h 35 Cl 2 CoN 2 OP) Calculated: C, 51.23; H, 7.17;...
Embodiment 3
[0163] Example 3: Catalytic Activity Experiment of Cobalt Complex IIa on the Asymmetric Hydrogenation of α-Alkenyl Silicon
[0164]
[0165] Take the asymmetric hydrogenation process of α-diphenylsilylstyrene VIIa as an example:
[0166] N 2 In the glove box, successively weigh catalyst IIa (0.01mmol, 4.9mg), n-pentane (2mL), α-diphenylsilylstyrene VIIa (0.2mmol, 57mg) in the reaction tube, and then slowly drop Add NaBHEt 3 (20μL, 0.02mmol, 1.0M in THF), the reaction system turned dark purple, and the reaction tube was placed in an autoclave, 2bar hydrogen gas was introduced, and the reaction was carried out at room temperature for 8h. The reaction was stopped, and the reaction system was exposed to air atmosphere to quench the reaction. It was then concentrated and purified by column chromatography (pure petroleum ether as eluent) to obtain Compound VIIIa as a colorless oil.
[0167] Using the above operation, different solvents, H 2 The pressure, the consumption of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com