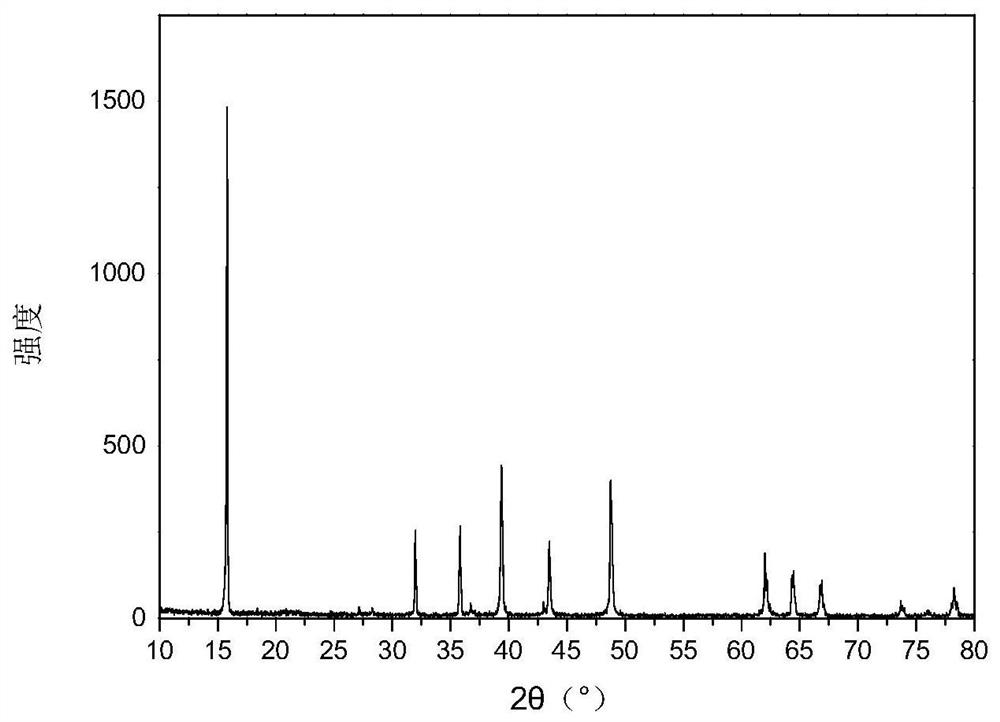
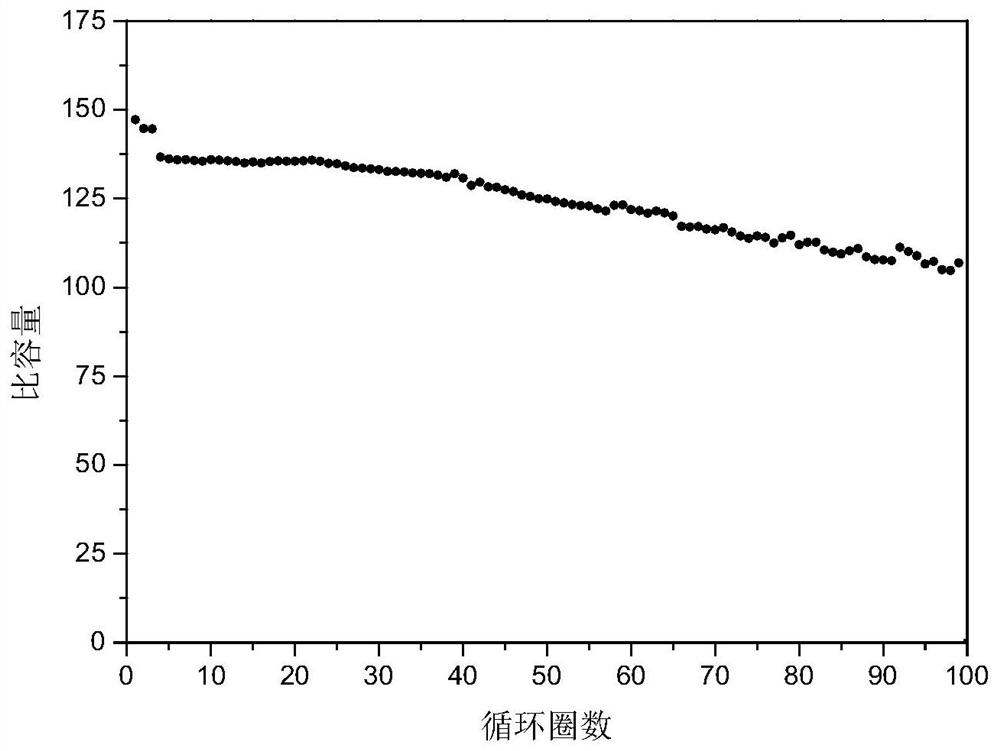
A diverse positive material and preparation method of a sodium ion battery
A technology for sodium ion batteries and positive electrode materials, which is applied in battery electrodes, positive electrodes, electrical components, etc., can solve problems such as low gram capacity and low stability, and achieve the effects of electrochemical performance modification, stable performance and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) According to the ratio of Na:Mn:Ni:Mg:W:B=0.67:0.7:0.15:0.1:0.05:0.03, Na carbonate is weighed 0.3664g, manganese acetate 1.7329g, nickel acetate 0.3770 g, 0.259g of magnesium nitrate, 0.1251g of ammonium tungstate, and 0.0186g of boric acid were dissolved in 50mL of deionized water, stirred and fully dissolved to form a uniform mixed solution.
[0036] (2) Weigh 2.112g of citric acid and add it into the mixed solution, and keep stirring in a 90°C water bath until the water evaporates to form a uniform sol.
[0037] (3) Put the sol in an oven at 120°C to dry the water, grind it into powder and put it in a muffle furnace for calcination, keep it at 450°C for 6 hours, then keep it at 900°C for 12 hours, then cool it naturally to get Na 0.67 mn 0.7 Ni 0.15 Mg 0.1 W 0.05 B 0.03 o 1.97 .
[0038] Weigh 0.07g of the above-prepared product, 0.02g of acetylene black (conductive agent), and 0.01g of PVDF (HSV900, binder). After fully grinding, add 0.6mL of NMP to disp...
Embodiment 2
[0043](1) According to the ratio of Na:Mn:Ni:Mg:W:B=0.67:0.7:0.15:0.1:0.05:0.05, Na excess 3% weighed 0.5924g of sodium nitrate, 1.7329g of manganese acetate and 0.3770g of nickel acetate g, 0.259g of magnesium nitrate, 0.1251g of ammonium tungstate, and 0.0311g of boric acid were dissolved in 50mL of deionized water, stirred and fully dissolved to form a uniform mixed solution.
[0044] (2) Weigh 2.112g of citric acid into the mixed solution, and keep stirring in a 90°C water bath until the water evaporates into a sol.
[0045] (3) Put the sol in an oven at 100°C to dry the water, grind it into powder and put it into a muffle furnace for calcination, keep it at 5000°C for 6 hours, then keep it at 925°C for 12 hours, then cool it naturally to get Na 0.67 mn 0.7 Ni 0.15 Mg 0.1 W 0.05 B 0.05 o 2 .
[0046] Weigh 0.07g of the above-prepared product, 0.02g of acetylene black (conductive agent), and 0.01g of PVDF (HSV900, binder). After fully grinding, add 0.6mL of NMP to di...
Embodiment 3
[0048] (1) According to the ratio of Na:Mn:Ni:Mg:W:B=0.67:0.7:0.2:0.05:0.05:0.03, Na carbonate is weighed 0.3736g, manganese acetate 1.7329g, nickel acetate 0.5027 g, 0.1259g of magnesium nitrate, 0.1251g of ammonium tungstate, and 0.0186g of boric acid were dissolved in 50mL of deionized water, stirred and fully dissolved to form a uniform mixed solution.
[0049] (2) Weigh 2.112g of citric acid and add it into the mixed solution, and keep stirring in a 90°C water bath until the water evaporates into a sol.
[0050] (3) Put the sol in an oven at 120°C to dry the water, grind it into powder and put it in a muffle furnace for calcination, keep it at 450°C for 6 hours, then keep it at 950°C for 10 hours, then cool it naturally to get Na 0.67 mn 0.7 Ni 0.2 Mg 0.05 W 0.05 B 0.03 o 2 .
[0051] Weigh 0.07g of the above-prepared product, 0.02g of acetylene black (conductive agent), and 0.01g of PVDF (HSV900, binder). After fully grinding, add 0.6mL of NMP to disperse and mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com