Drug-loaded delivery drug delivery system for treating systemic lupus erythematosus and preparation method thereof
A lupus erythematosus, systemic technology, applied in the field of biomedicine, can solve the problems of difficult transfection, tumorigenicity, viral vector side effects, immunogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0112] I. Experimental method:
[0113] 1. Characterization of Nanoparticles
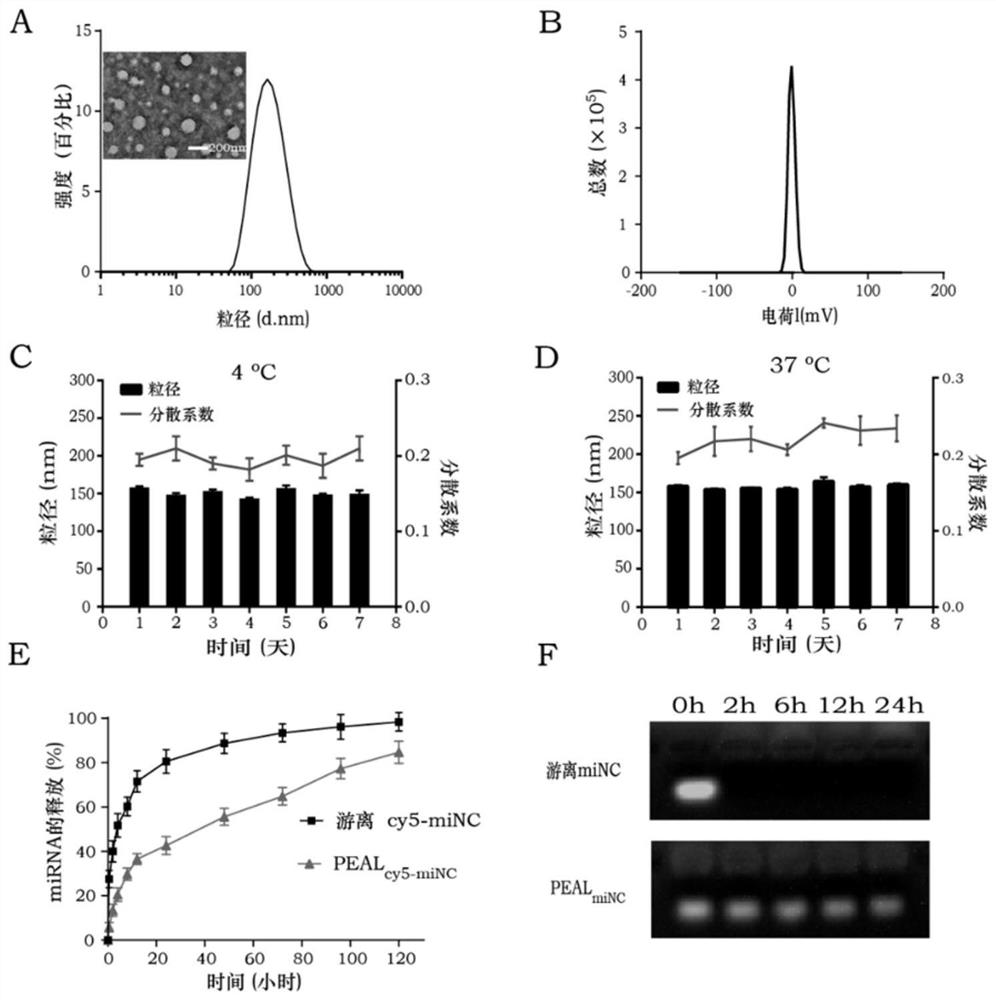
[0114] Illustratively, the following Example 1 was used to prepare PEALmiR125a nanoparticles for subsequent experiments, and the Nano-zs particle size analyzer was used to measure the particle size and potential of the nanoparticles. The transmission electron microscope (TEM) was used to observe the structure of the nanoparticles. NanoDrop2000 was used to determine the encapsulation efficiency of nanoparticles. Specifically, the prepared PEALmiR125a was ultracentrifuged, the parameters were set to: 15000 g, 30 minutes, and the supernatant was taken. NanoDrop 2000 was used to determine the concentration of RNA in the stock solution and supernatant of nanoparticles. Then calculate the encapsulation rate according to the following formula. Encapsulation rate (%)=(1-amount of RNA in supernatant / amount of RNA in initial stock solution)×100%. The solutions were placed at 37°C and 4°C, samples were taken ...
Embodiment 1
[0149] Example 1 Construction and application of mPEG-PLGA-PLL nano-delivery system containing miR-125a
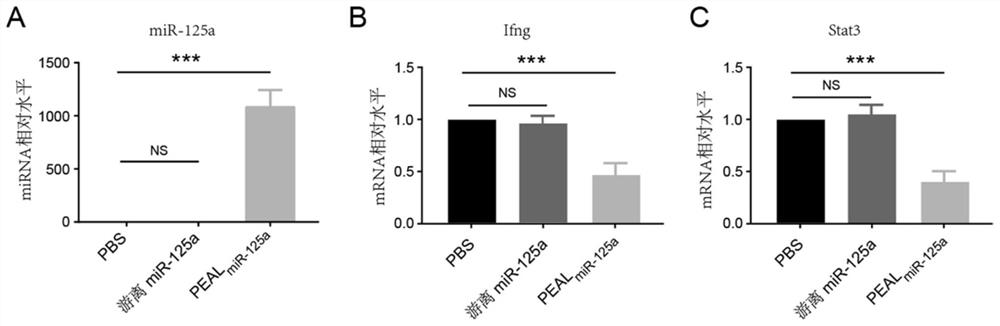
[0150] Preparation by emulsification evaporation method: accurately weigh 10mg of mPEG-PLGA-PLL and dissolve it in 500μl of dichloromethane, then add 5nmol of miR-125a diluted in DEPC water and mix ultrasonically (2S, 2S, 300W) under ice bath conditions to make Colostrum, then quickly pour it into 5ml of F68 aqueous solution (1%, w / v) aqueous solution, ultrasonic emulsification again under ice bath conditions, vacuum rotary evaporation to remove organic solvents in the preparation, that is, miR-125a / PEAL(PEAL miR-125a ) Nanoparticles.
[0151] The obtained nanoparticles have a diameter of about 158nm and a surface charge of +0.01mV. And the nanoparticle size and dispersion coefficient did not change significantly within a week at 4°C and 37°C, showing good stability.
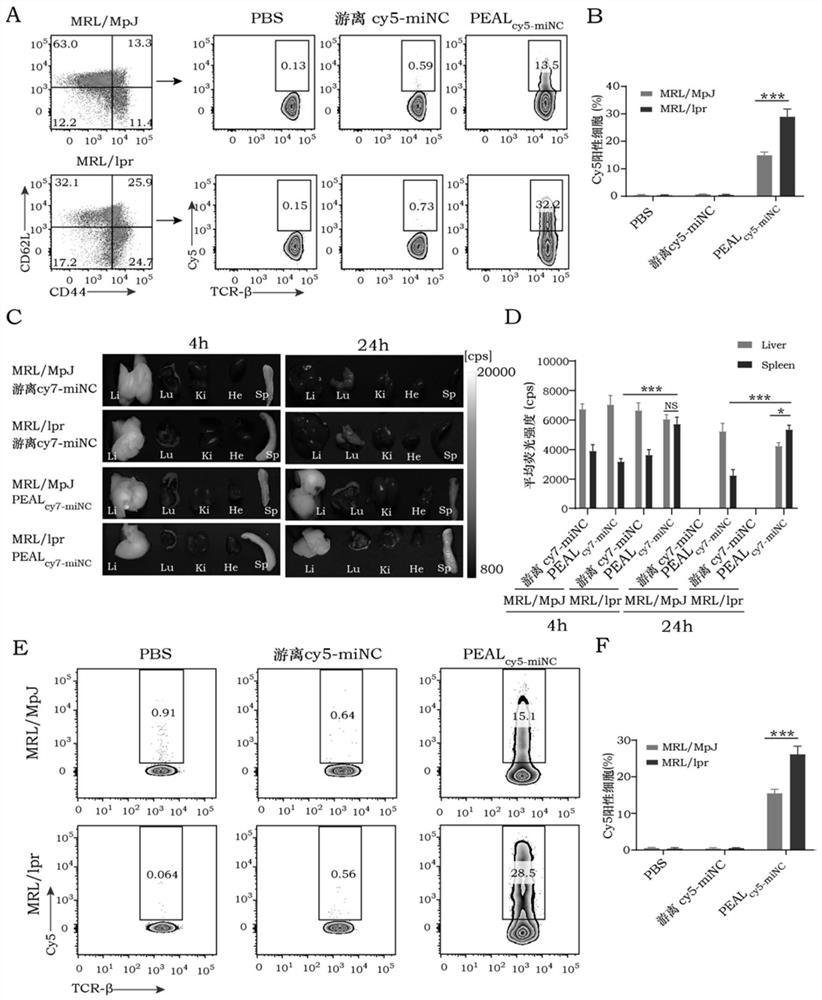
[0152] Select 10-week-old MRL / lpr mice of spontaneous systemic lupus erythematosus model and divide them in...
Embodiment 2
[0153] Example 2: Construction and application of mPEG-PLGA-PLL nano delivery system containing miR-146a
[0154] Volumetric volatilization method: accurately weigh 10mg mPEG-PLGA-PLL, dissolve it in 1ml tetrahydrofuran, and then inject it into 5ml DEPC water containing 5nmol miR-146a. Stir until the tetrahydrofuran volatilizes to obtain the nanoparticle solution (PEALmiR-146a).
[0155] Using the MRL / lpr mouse model, referring to Example 1, PEALmiR-146a (miR-146a dose of 1.5 mg / kg) nanoparticles were injected into the mice through the tail vein twice a week for 4 weeks. PBS and free miR-146a served as controls. The results showed that PEALmiR-146a treatment significantly reduced spleen and lymph node enlargement, reduced proteinuria, and improved lupus nephritis (P<0.05).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com