Quality control product for novel coronavirus detection kit and preparation method thereof
A technology for detection kits and coronaviruses, which is applied in the field of preparation of quality control products for novel coronavirus fluorescence quantitative detection kits, which can solve problems such as delayed disease, individual misjudgments, false negatives, etc., to ensure effective storage and prolong storage time , to ensure the effect of detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] ——Quality control product IBV QXL87 strain virus content and purity detection
[0042] According to the third part of the 2015 edition of the Veterinary Pharmacopoeia of the People's Republic of China, the purity of the virus seeds of the QXL87 strain was tested to determine the purity of the virus seeds.
[0043] Carry out virus content determination to virus seed according to the following method, QXL87 strain chicken embryo allantoic fluid is made 10 times of serial dilutions with sterile normal saline, takes 10 -5 、10 –6 、10 –7 、10 –8 For 4 dilutions, 5 10-day-old SPF chicken embryos were inoculated into each allantoic cavity, 0.1ml per embryo, and incubated at 36-37°C. Chicken embryos that died before 24 hours were discarded, and chicken embryos that died within 24 to 144 hours were taken out at any time, until 144 hours, all live embryos were taken out. 24-144 hours after the inoculation, the dead chicken embryos and the surviving chicken embryos showed speci...
Embodiment 2
[0048] ——Quality control product IBV QXL87 strain S1 gene identification strain
[0049] According to the sequence of chicken infectious bronchitis virus (IBV) S1 gene included in GenBank, primers were designed for the conserved region after comparison, and were synthesized by Shanghai Sangon Bioengineering Co., Ltd.
[0050] Sequence 1 upstream primer S1–F: 5′–atgttgggga agtcactgtt–3′20
[0051] Sequence 2 downstream primer S1–R: 5′–tgcgacgatg tgagctattg–3′20
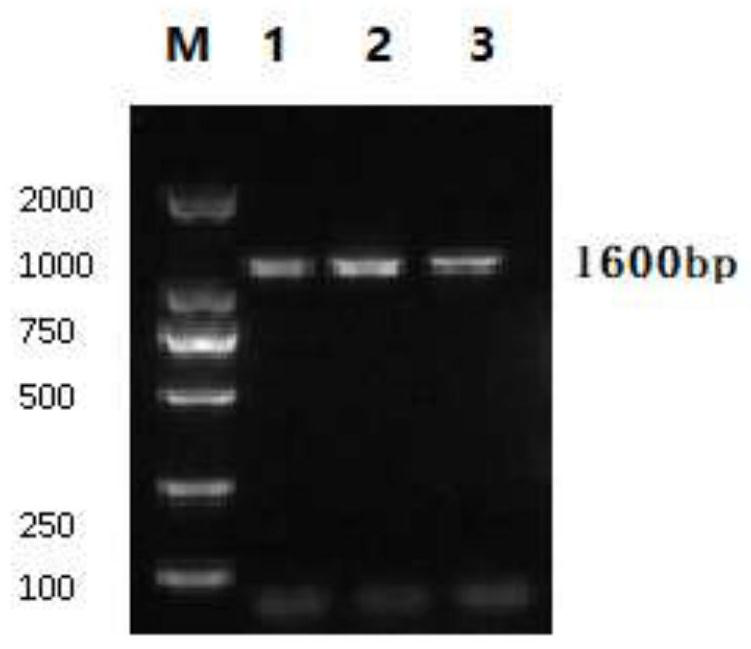
[0052] The IBV QXL87 strain was inoculated into 10-day-old SPF chicken embryos, and the allantoic fluid of 42-hour chicken embryos was collected. The S1 gene of QXL87 strain was amplified by RT-PCR, and the amplified product was cloned, sequenced, and compared with the gene sequence. Viral RNA was extracted from the allantoic fluid according to the instructions of the RNA extraction kit of Jiangsu Kangwei Century Biotechnology Co., Ltd. After reverse transcription (RT), RT-PCR amplification, electrophoresis and othe...
Embodiment 3
[0055] ——Quality control product IBV QXL87 strain fluorescence quantification
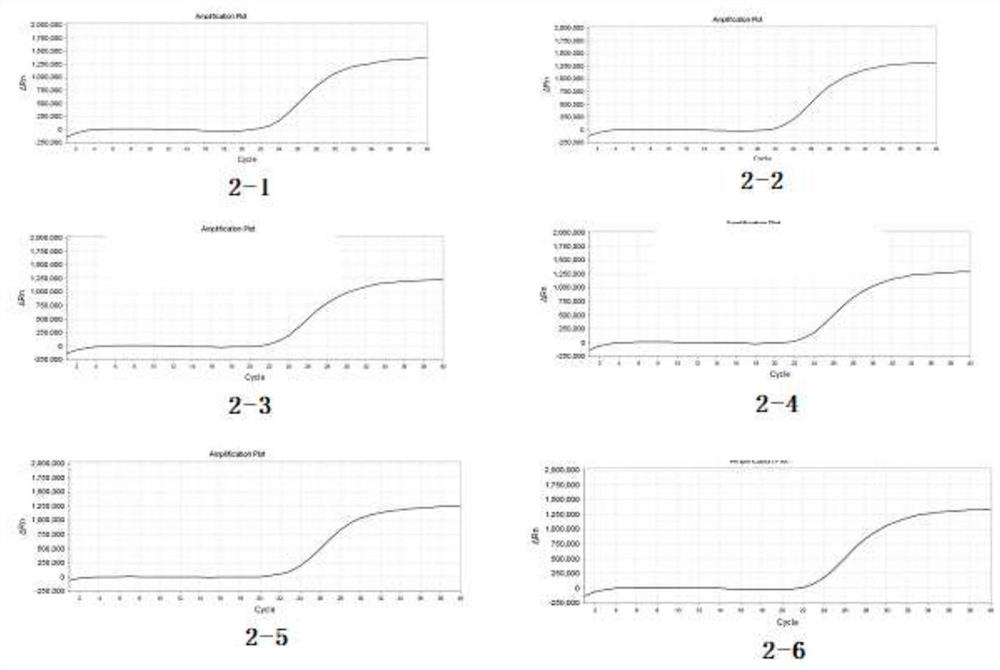
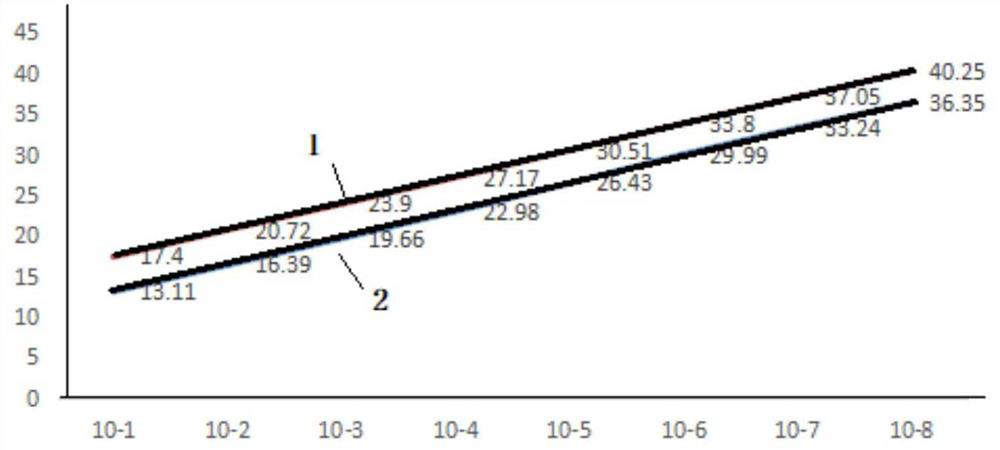
[0056] According to the sequence of the S1 gene of the QXL87 strain, specific primers and probes were designed using software, RNA was extracted using the RNA extraction kit of Jiangsu Kangwei Century Biotechnology Co., Ltd., and the virus species of the QXL87 strain was detected by one-step qPCR (TaqMan). Optimize detection conditions, analyze primer melting curves, verify the specificity and sensitivity of fluorescent probes and primers, and ensure the specificity of primers and fluorescent probes. The reaction system is a 25 μL system, including 19 μL of reaction solution (including specific primers, probes, and reaction buffer), 1 μL of enzyme solution (including reverse transcriptase, hot-start DNase, etc.), and 5 μL of sample to be tested.
[0057] Sequence 1 upstream primer S1–F: 5′–ctgttcgatt agtcactgtt–3′20
[0058] Sequence 2 downstream primer S1–R: 5′–tcctt cgatg tgagc caatt–3′20
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com