A biofilm for eye surface repair and its preparation method
A biofilm and ocular surface technology, applied in the field of tissue engineering medical biomaterials, can solve problems such as hidden dangers of virus transmission, difficult removal of amniotic membrane, loss of natural collagen, etc. effect of structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
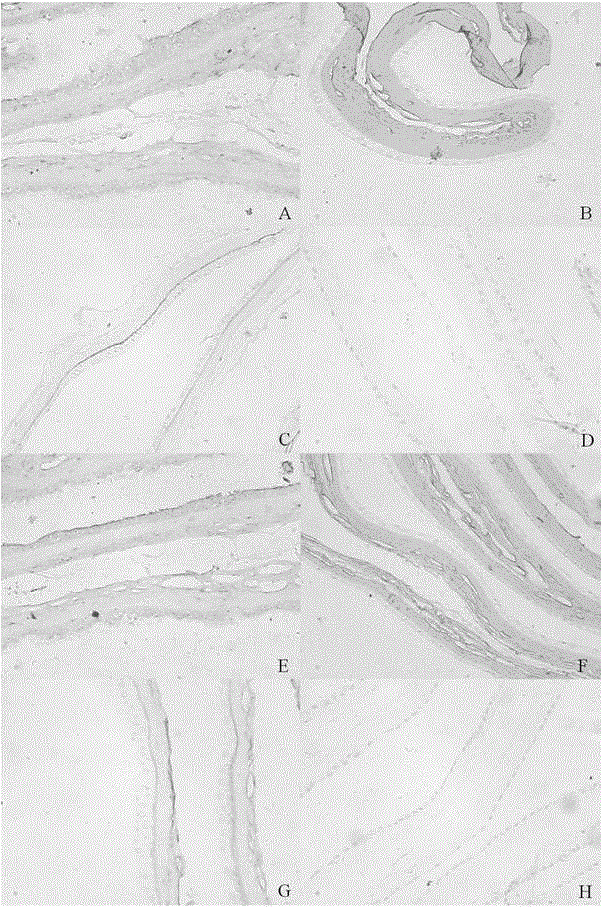
[0040] Step 1. Disinfection of raw materials: wash the amniotic membrane with physiological saline for 3 times, soak in 0.5M NaOH aqueous solution for 0.5 hour, and then wash with purified water for 3 times;
[0041] Step 2, antibacterial treatment: soak the sterilized amniotic membrane in physiological saline containing 50 μg / mL penicillin and 50 μg / mL streptomycin for 15 minutes;
[0042]Step 3, protection treatment: soak the amniotic membrane treated in step 2 in the mixed protection solution and shake for 24 hours; the mixed protection solution is a 1:1 mixture of dry protection solution and radiation protection solution by volume; the dry protection solution is 1g / L Trehalose aqueous solution of concentration; Radiation protection liquid is the ascorbic acid aqueous solution of 5g / L concentration;
[0043] Step 4. Vacuum drying: vacuum-dry the amniotic membrane treated in step 3, the vacuum degree is 0.1Mpa, and the temperature is controlled at 40°C, and the drying time i...
Embodiment 2
[0047] Step 1. Disinfection of raw materials: wash the amniotic membrane with normal saline for 5 times, soak in 75% alcohol solution for 5 hours, and then wash with purified water for 10 times;
[0048] Step 2, antibacterial treatment: soak the sterilized amniotic membrane in phosphate buffer solution with pH 7.0 containing 4 μg / mL gentamicin and 2.5 μg / mL amphotericin B for 1 hour;
[0049] Step 3, protection treatment: soak the amniotic membrane treated in step 2 in the mixed protection solution and shake for 1 hour; the mixed protection solution is a dry protection solution mixed with a radiation protection solution at a volume ratio of 1:2; wherein the dry protection solution has a volume concentration of 40% glycerol aqueous solution; the radioprotectant is 10g / L mannitol aqueous solution;
[0050] Step 4. Vacuum drying: the amniotic membrane treated in step 3 is vacuum-dried with a vacuum degree of -0.08Mpa, the temperature is controlled at 55°C, and the drying time is ...
Embodiment 3
[0054] Step 1. Disinfection of raw materials: wash the amniotic membrane 4 times with normal saline, soak in 1g / L hydrogen peroxide aqueous solution for 1 hour, and then wash 6 times with purified water;
[0055] Step 2, antibacterial treatment: soak the sterilized amniotic membrane in physiological saline containing 50 μg / mL penicillin and 2.5 μg / mL amphotericin B for 30 minutes;
[0056] Step 3, protection treatment: soak the amniotic membrane treated in step 2 in the mixed protection solution and shake for 24 hours; the mixed protection solution is a mixture of dry protection solution and radiation protection solution at a volume ratio of 1:1.5; the dry protection solution is 15g / L trehalose aqueous solution; radiation protection solution is 5g / L tea polyphenol aqueous solution.
[0057] Step 4. Vacuum drying: the amniotic membrane treated in step 3 is vacuum-dried with a vacuum degree of 0.05 Mpa, and the temperature is controlled at 45° C. for 2 hours.
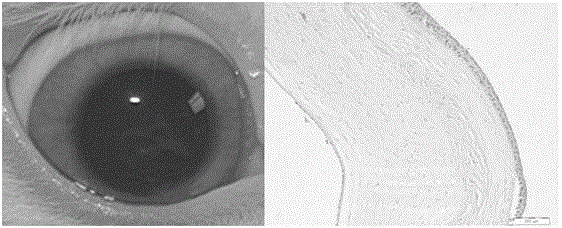
[0058] Step 5, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com