Statin and vitamin D composition and preparation method and application thereof
A composition and vitamin technology, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc., can solve problems such as rhabdomyolysis, and achieve the effects of improved solubility, small and uniform particle size, and strong drug-carrying capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment approach
[0027] One implementation is:
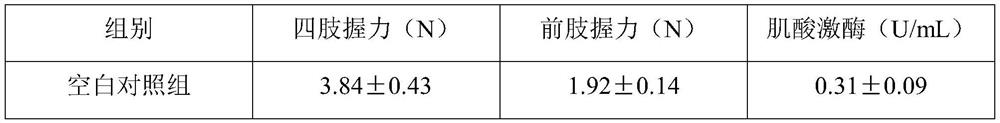
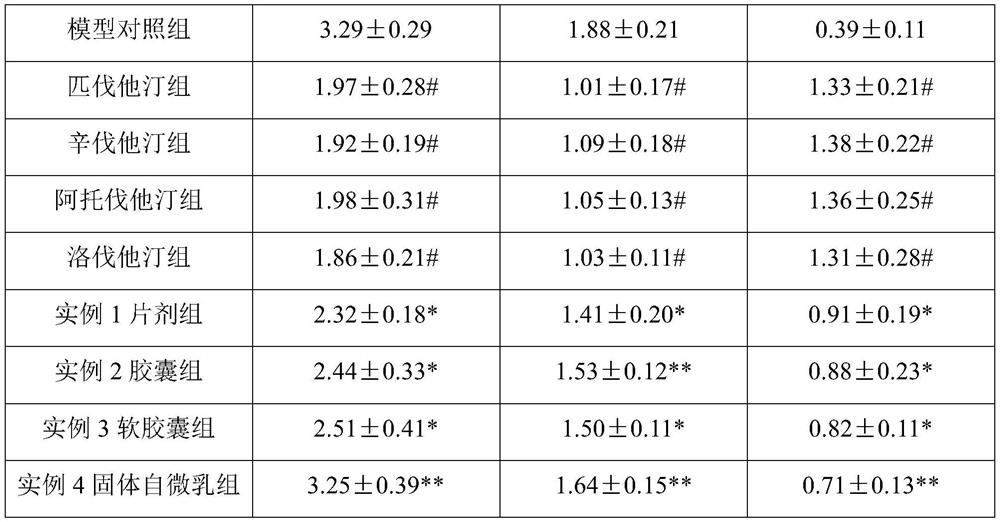
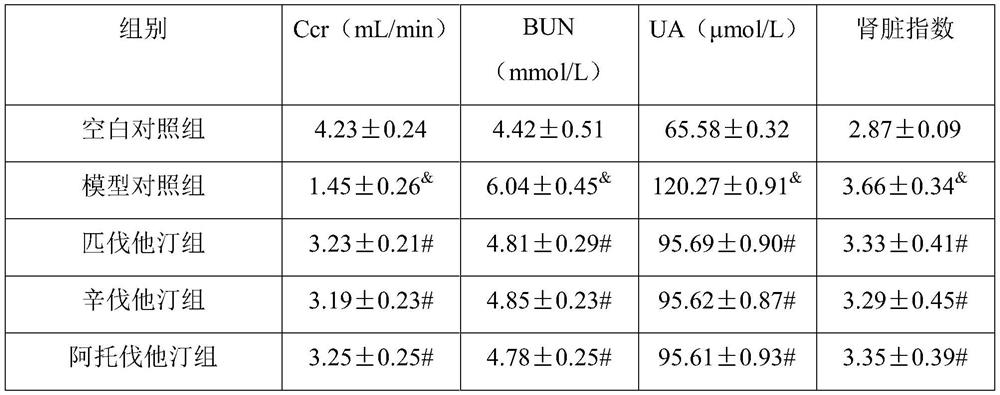
[0028] The composition includes: a statin composition and vitamin D as active ingredients, wherein the material ratio of the two is 1-80 mg of the statin composition and 1000-50000 units of vitamin D.
[0029] Wherein, the statin composition includes lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, rosuvastatin or pitavastatin; vitamin D includes vitamin D2 and / or vitamin D3.
[0030] Another embodiment is: a composition, including: a statin composition and vitamin D as active ingredients, and also includes: an oil phase, a surfactant, a co-surfactant, and a solid adsorbent, wherein the material ratio of the two is The statin composition is 1-80 mg, the vitamin D is 1000-50000 units, and the weight ratio of oil phase, surfactant, co-surfactant and solid adsorbent is 1:1-2:1-3:2-3.
[0031] The statin drug and vitamin D are mixed and then added to the oil phase to stir and dissolve to form an oil phase system. The surfactant and ...
example 1
[0034] Composition, prepared into dispersible tablets;
[0035] Dissolve sodium carboxymethyl cellulose in an appropriate amount of distilled water and stir until gelatinous. Weigh the prescription amount of statin, vitamin D3 composition and starch to mix, add carboxymethyl cellulose sodium solution and mix to prepare soft material, sieve the prepared soft material by extrusion method, and then dry it in an oven at 30°C , after sieving the dry granules and adding sodium carboxymethyl starch for mixing, adding talcum powder and mixing evenly, and performing manual tablet compression with a tablet machine to prepare solid dispersible tablets of the composition of statin and vitamin D3.
[0036] Appropriate amount of statin × 1000, vitamin D3 2000 units × 1000, sodium carboxymethylcellulose 0.5g, talcum powder 0.3g, starch 12.7g, appropriate amount of water, made into 1000 tablets.
[0037] The dosage ratio of each dispersible tablet containing statins and vitamin D3:
[0038]...
example 2
[0040] Composition, prepared into capsules;
[0041] Weigh the prescribed amount of statin and vitamin D2 composition, microcrystalline cellulose, and cross-linked carboxymethyl cellulose, mix evenly for 30 minutes, add an appropriate amount of 2% hypromellose aqueous solution to make a soft material, pass through a 24-mesh sieve to granulate , dried at 60°C, the dry granules passed through a 24-mesh sieve for granulation, added the prescribed amount of talcum powder and mixed evenly, measured the content of intermediates, calculated the filling volume, and filled with 2# capsules.
[0042] Appropriate amount of statin × 1000, vitamin D2 5000 units × 1000, microcrystalline cellulose 14g, croscarmellose 5g, talcum powder 1g, hypromellose solution (2%) appropriate amount, made into 1000 capsules.
[0043] The dosage ratio of statins and vitamin D2 per capsule:
[0044] Simvastatin (SIM, Simvastatin) 10mg; vitamin D2 5000 units.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com