Etamsylate gel patch and preparation method thereof
A technology of acetamine gel patch and acetamine, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of not being able to give priority to guaranteeing Stability, not suitable for daily drug administration, can not be exposed at any time, etc., to achieve the effect of reducing systemic adverse reactions, reducing systemic adverse reactions, and not easy to stain clothes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
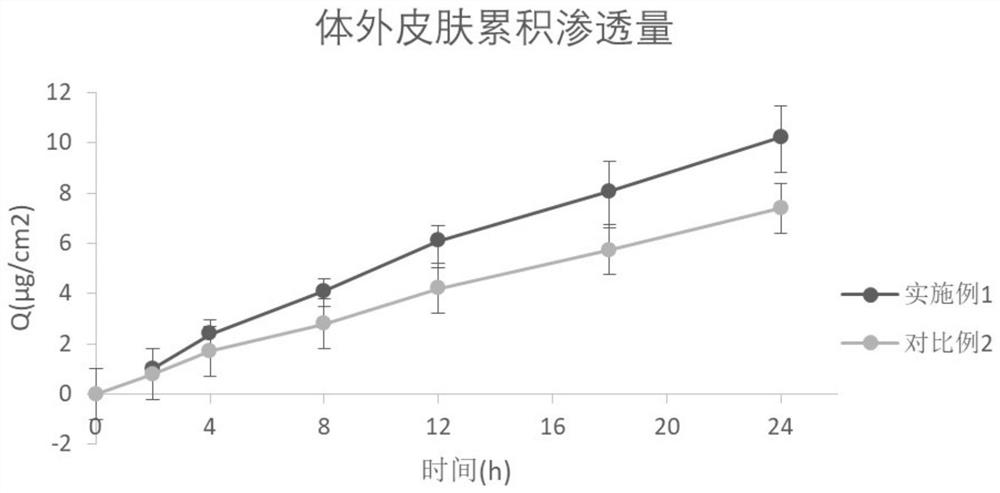
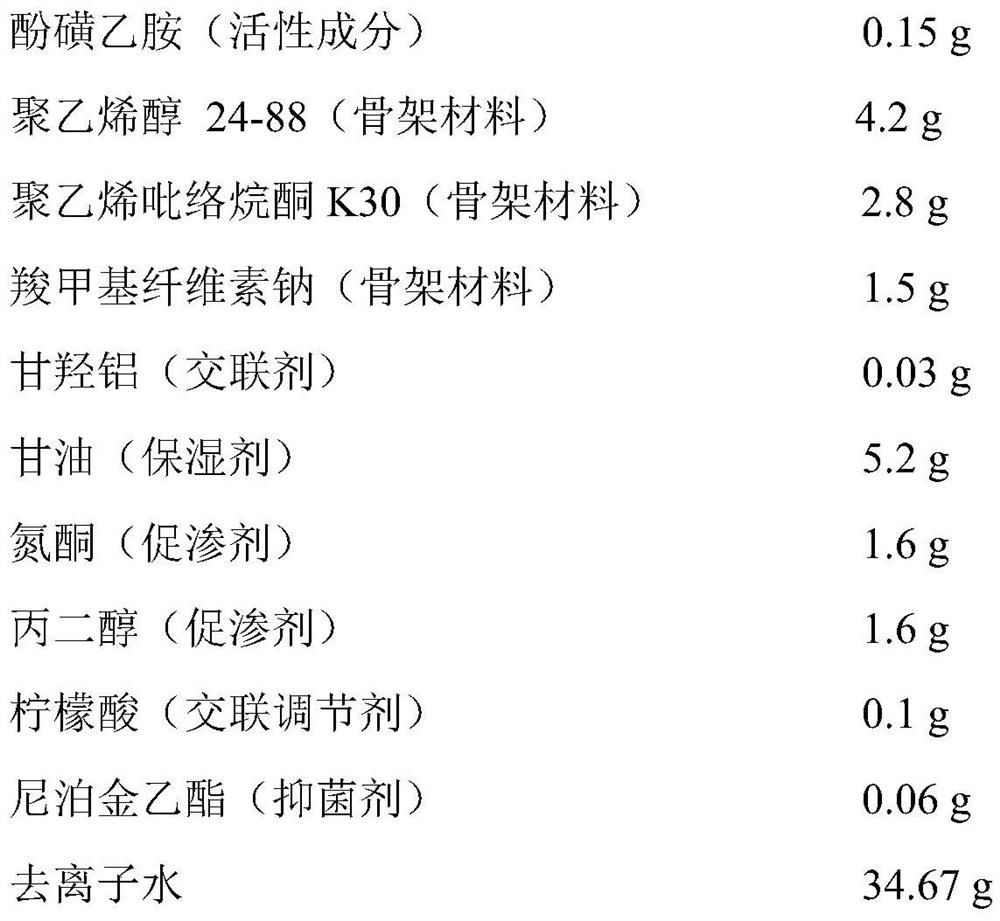
[0027] Embodiment 1 prepares phensulfame gel patch
[0028] Drug-containing gel matrix components (per 100cm 2 )
[0029]
[0030] Preparation:
[0031] (1) Drug-containing gel base: Add the prescribed amount of polyvinyl alcohol, polyvinylpyrrolidone K30, sodium carboxymethylcellulose, aluminum glycylate, azone, propylene glycol, and ethylparaben into glycerin, and stir Mix evenly, and use it as phase I; add the prescription amount of phensulfame and citric acid into deionized water to dissolve, and use it as phase II; add phase I to phase II, and stir rapidly to obtain a drug-containing gel matrix;
[0032] (2) Adhesive layer preparation: evenly coat the water-based acrylic adhesive 829A forming the adhesive layer on the backing layer, the coating weight is 8.5% of the weight of the backing layer, dry at 55-85°C, and cool for later use;
[0033] (3) Add the drug-containing gel base to the adhesive layer, cover with an anti-adhesive layer, cut and pack, and the product ...
Embodiment 2
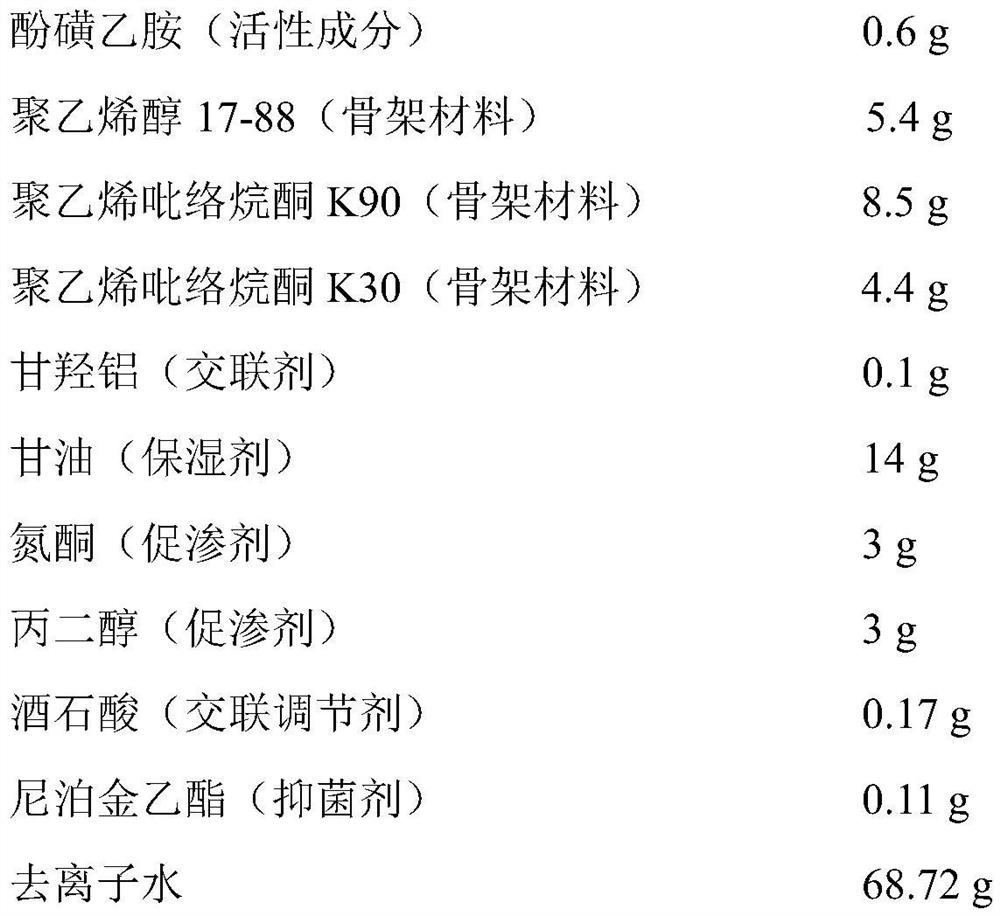
[0034] Embodiment 2 prepares phensulfame gel patch
[0035] Drug-containing gel matrix components (per 100cm 2 )
[0036]
[0037] Preparation method: (1) Drug-containing gel base: add the prescribed amount of polyvinyl alcohol, polyvinylpyrrolidone K90, polyvinylpyrrolidone K30, aluminum glycylate, azone, propylene glycol, and ethylparaben In glycerin, stir and mix evenly, as phase I; add the prescribed amount of ethanol and tartaric acid into deionized water to dissolve, as phase II; add phase I to phase II, and stir quickly to obtain the drug-containing gel matrix;
[0038] (2) Adhesive layer preparation: evenly coat the water-based acrylic adhesive 829A forming the adhesive layer on the backing layer, the coating weight is 14.5% of the weight of the backing layer, dry at 55-85°C, and cool for later use;
[0039] (3) Add the drug-containing gel base to the adhesive layer, cover with an anti-adhesive layer, cut and pack, and the product is obtained.
Embodiment 3
[0040] Embodiment 3 prepares phensulfame gel patch
[0041] Drug-containing gel matrix components (per 100cm 2 )
[0042]
[0043] Preparation method: (1) Drug-containing gel base: Add the prescribed amount of polyvinyl alcohol, gelatin, carbomer, aluminum glyoxate, azone, propylene glycol, and ethyl paraben into glycerin, stir and mix evenly, and use it as phase I ; Dissolve the prescribed amount of ethylamine sulfate and tartaric acid in deionized water as phase II; add phase I to phase II and stir rapidly to obtain a drug-containing gel matrix;
[0044] (2) Adhesive layer preparation: evenly coat the water-based acrylic adhesive 829A forming the adhesive layer on the backing layer, the coating weight is 13.7% of the weight of the backing layer, dry at 55-85°C, and cool for later use;
[0045] (3) Add the drug-containing gel base to the adhesive layer, cover with an anti-adhesive layer, cut and pack, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com