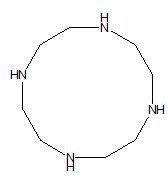
Novel synthesis method of 1, 4, 7, 10-tetraazacyclododecane
A synthesis method and technology of tetranitrogen ring, applied in the direction of organic chemistry, etc., can solve the problems of low total molar yield, long process route, high production cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-1
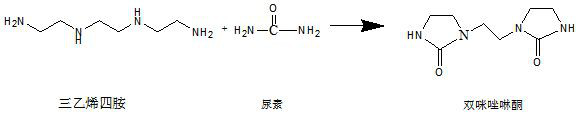
[0040] Put 146g of triethylenetetramine (originally produced in Tosoh, Japan) (1mol) and 120g of urea (2mol) into a 2000ml reaction bottle equipped with a 300W motor stirring, insert a thermometer and connect the gas outlet to the exhaust gas treatment device. After the stirring is turned on, use a heating mantle to raise the temperature. When the temperature reaches 130°C, the material in the bottle suddenly expands and a large amount of ammonia gas escapes. Continue to heat up and keep warm at 150°C for half an hour. As the heat preservation proceeds, the material in the bottle gradually becomes dry powder, and the escaped gas gradually decreases until no more gas escapes. Remove the electric heating mantle, stir and drop to room temperature.
[0041] Add 1000ml of methanol aqueous solution with a mass fraction of 50% to the bottle, connect the tail gas port to a reflux condenser, and heat to boiling reflux in a water bath while stirring, and the reflux temperature is 64°C...
example 1-2
[0043] Put 146g of triethylenetetramine (originally produced in Tosoh, Japan) (1mol) and 132g of urea (2.2mol) into a 2000ml reaction bottle equipped with a 300W motor stirring, insert a thermometer and connect the gas outlet to the exhaust gas treatment device. After the stirring is turned on, use a heating mantle to raise the temperature. When the temperature reaches 130°C, the material in the bottle suddenly expands and a large amount of ammonia gas escapes. Continue to heat up and keep the temperature at 200°C for 10 minutes. As the heat preservation proceeds, the material in the bottle gradually becomes dry powder, and the escaped gas gradually decreases until no more gas escapes. Remove the electric heating mantle, stir and drop to room temperature.
[0044]Add 1000ml of methanol aqueous solution with a mass fraction of 50% to the bottle, connect the tail gas port to a reflux condenser, and heat to boiling reflux in a water bath while stirring, and the reflux temperatu...
example 2-1
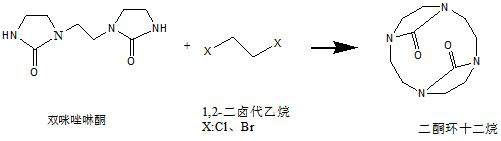
[0047] Weigh 20 g of the bis-imidazolinone (0.1 mol) obtained in Example 1-1 and add it into 200 ml of ethyl acetate, stir and dissolve for later use.
[0048] Add 15g of 1,2-dichloroethane (0.15mol), 107g of cesium carbonate (0.33mol) and 800ml of ethyl acetate into a 2000ml flask equipped with a thermometer, dropping funnel and stirring, and cool with a dry ice acetone bath. When the temperature dropped to minus 30°C, the ethyl acetate solution of the aforementioned bis-imidazolidinone was added dropwise. Adjust the rate of addition so that the temperature of the system does not exceed minus 10°C. After the dropwise addition, keep the temperature between minus 10°C and 0°C for 5 hours.
[0049] After the heat preservation was completed, the reaction solution was poured out and filtered, and the filtrate was concentrated under reduced pressure to a volume of 100ml, and the concentrated solution was placed in a refrigerator to freeze overnight. The next day, flaky crystals w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com