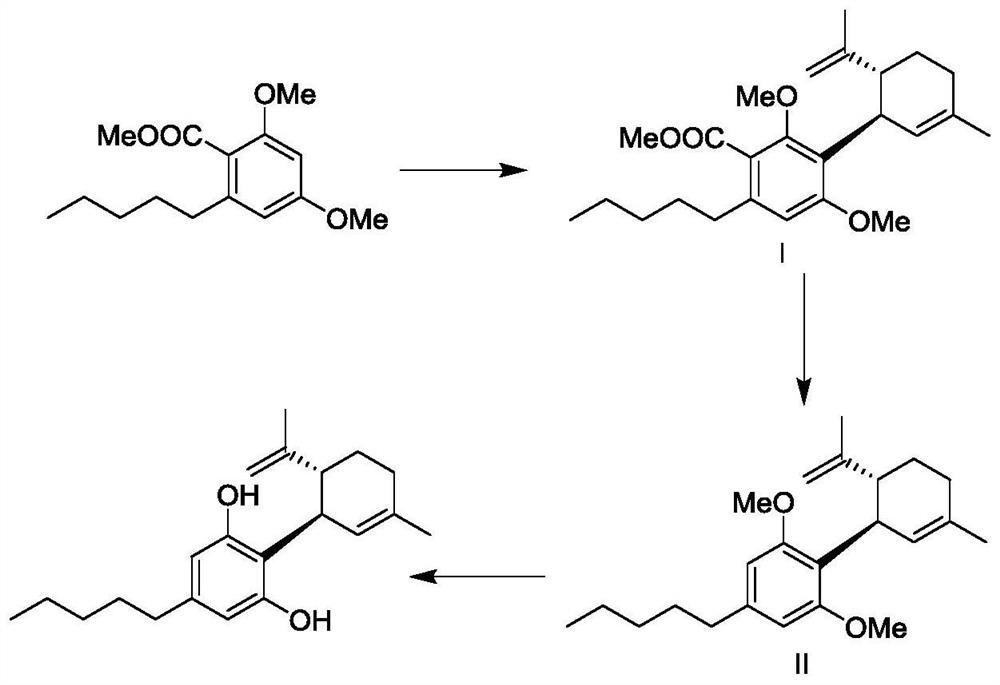
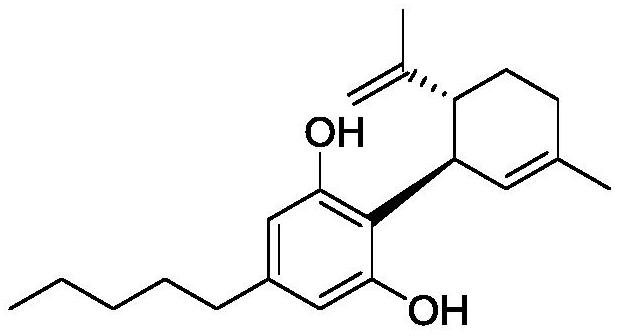
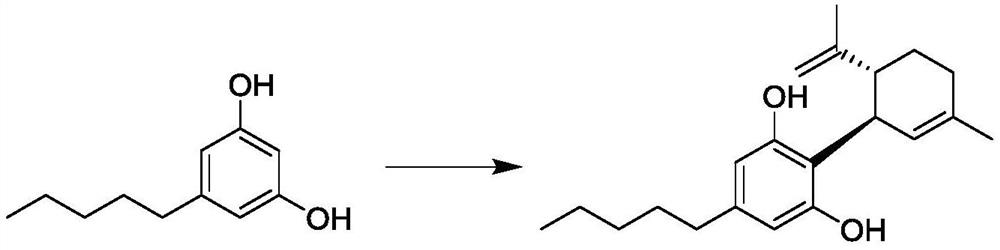
Synthetic method of cannabidiol
A technology for cannabidiol and a synthesis method, which is applied in the directions of organic chemistry methods, chemical instruments and methods, active ingredients of hydroxyl compounds, etc., can solve problems such as large limitations in industrialized production, cumbersome steps, etc., and achieves good industrial application prospects and process improvement. , the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of intermediate one:
[0047] Add 100g of methyl 2,4-dimethoxy-6-pentylbenzoate and 800mL of dichloromethane into a 2000mL three-neck flask, add 50ml of boron trifluoride ether under stirring, stir at 25°C for 0.5 hours, drop 50g of (1S,4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexen-1-ol was added dropwise and kept stirring for 24 hours. Cool the reaction solution below 10°C, adjust the pH to 2-3 with 1N hydrochloric acid aqueous solution, add n-heptane (500mL×2) for extraction, adjust the pH to 10 with solid sodium carbonate in the aqueous phase, add n-heptane (500mL×2) Extracted, washed with water (300 mL) once, dried over anhydrous sodium sulfate, concentrated under reduced pressure to dryness to obtain a crude product. Add 4 v / m n-heptane to the crude product and heat to dissolve at 40°C, cool down to -5-5°C for 16 hours, keep warm and crystallize, filter with suction, and dry to obtain 81g of white solid with a yield of 73% and a purity of 99.96% by HPLC....
Embodiment 2
[0053] Preparation of intermediate one:
[0054] Add 100g of methyl 2,4-dimethoxy-6-pentylbenzoate and 300mL of DMF into a 1000mL three-necked flask, add 40ml of boron trifluoride ether under stirring, stir at 25°C for 0.5 hours, and dropwise add 70g of (1S,4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexen-1-ol, after the dropwise addition, keep stirring for 15 hours. Cool the reaction solution below 0°C, adjust the pH to 2-3 with 1N dilute sulfuric acid aqueous solution, add n-heptane (500mL×2) for extraction, adjust the pH to 10 with solid sodium carbonate in the aqueous phase, add n-heptane (500mL×3 ) extraction, washed with water (300mL); twice, dried over anhydrous sodium sulfate, concentrated under reduced pressure to dryness to obtain a crude product. The crude product was dissolved by adding 4 v / m n-heptane at 40°C, cooled to -5-5°C for 16 hours, and then crystallized at -5°C for 16 hours. After suction filtration and drying, 75g of a white solid was obtained. The yield was...
Embodiment 3
[0060] Preparation of intermediate one:
[0061] Add 100g of methyl 2,4-dimethoxy-6-pentylbenzoate and 500mL of tetrahydrofuran into a 2000mL three-neck flask, add 50ml of boron trifluoride ether under stirring, stir at 25°C for 0.5 hours, and dropwise add 70g of (1S,4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexen-1-ol, after the dropwise addition, keep stirring for 18 hours. Cool the reaction solution below 10°C, adjust the pH to 2-3 with glacial acetic acid, add n-heptane (500mL×2) for extraction, adjust the pH to 10 with solid sodium carbonate in the aqueous phase, add n-heptane (500mL×3) for extraction , washed with water (300 mL) twice, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a crude product. Add 4 v / m n-heptane to the crude product and heat to dissolve at 40°C, cool down to -5-5°C for crystallization for 16 hours, filter with suction, and dry to obtain 79g of white solid with a yield of 71% and a purity of 99.92% by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com