Preparation method of metreleptin
A technology of metriptin and equilibration solution, applied in the field of protein engineering, can solve the problems of difficult industrialization and amplification, low yield, etc., and achieve the effects of high renaturation efficiency, high yield, and avoiding aggregation and precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Construction of Metreptine Recombinant Expression Vector
[0049] 1.1 Sequence optimization and synthesis of mettriptin expression gene
[0050] Referring to the prokaryotic Escherichia coli codon usage frequency table, combined with factors such as mRNA secondary structure stability and GC content balance, bases were optimized without changing the amino acid sequence of metreptine shown in SEQ ID NO.1 In order to maximize the expression of the target protein, the optimized mettriptin expression gene sequence is shown in SEQ ID NO.2. The sequence was synthesized by chemical synthesis.
[0051] 1.2 Construction of mettriptin recombinant expression vector
[0052] The prokaryotic expression vector was used to construct the recombinant expression vector of mettriptin. In this example, the recombinant expression vectors were all constructed by the method of seamless cloning. The target protein (metriptin) genes are all immediately after the start codon ATG, a...
Embodiment 2
[0087] Example 2: Construction and Induced Expression of Metreptine Genetic Engineering Bacteria
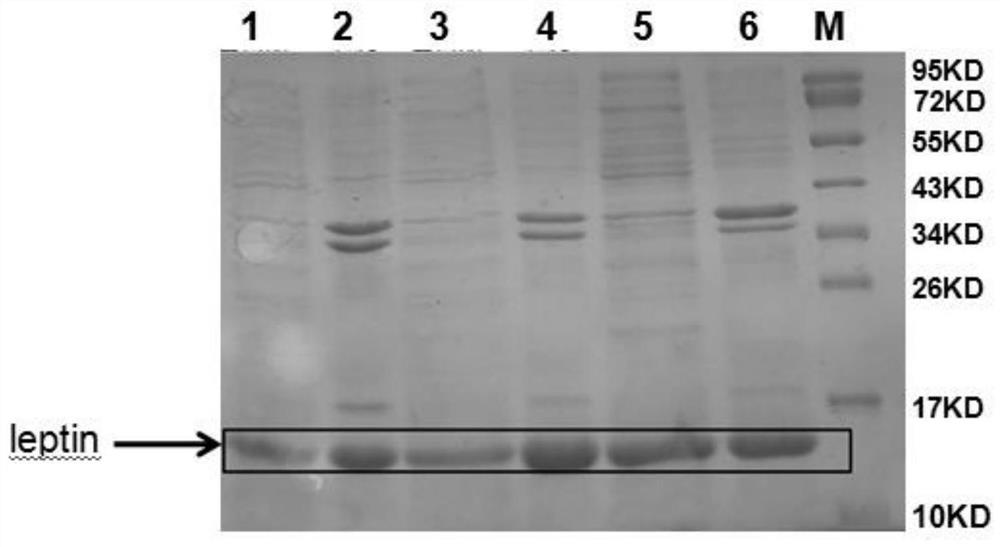
[0088] The recombinant expression vector constructed in Example 1.2 was passed through CaCl 2 Heat shock transformation method or electroporation method (Electroporation) is transferred to the adapted Escherichia coli expression strain cells, and the recombinants are obtained after screening, which is the genetic engineering strain. Listed below to illustrate:
[0089] 2.1 Construction and induced expression of pET-DE3 engineering strain
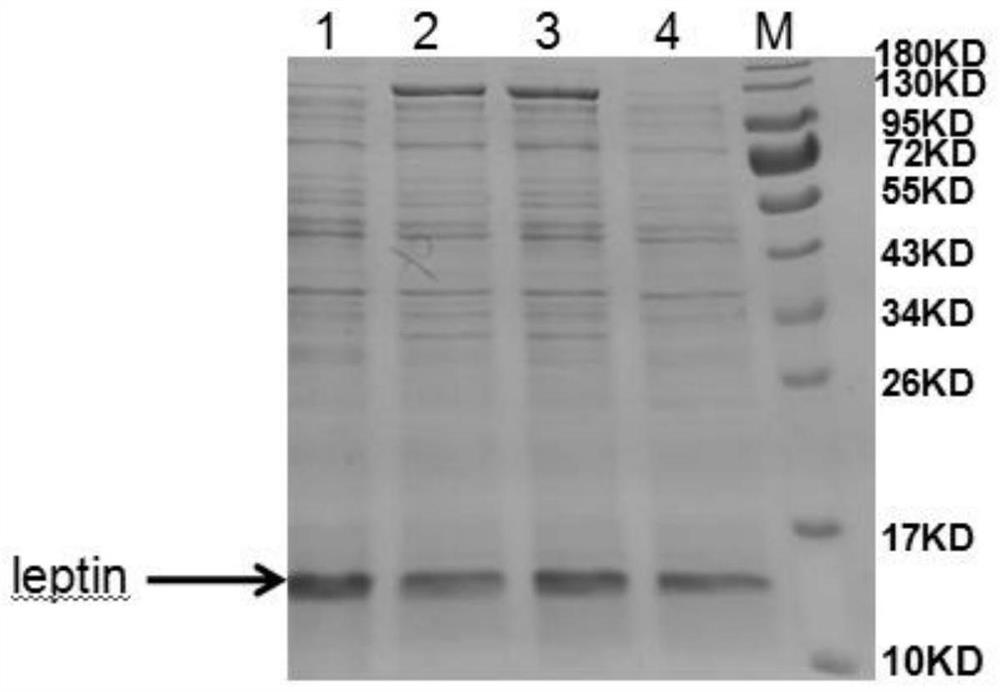
[0090] The pET32a(+)-leptin recombinant expression vector constructed in Example 1.2.1 was transformed into BL21(DE3) Escherichia coli expression strain, and cultured on LB solid medium containing ampicillin with a final concentration of 100 μg / ml at 37°C for 13~ After 16 hours of screening, the recombinant was obtained, which was the mettriptin genetically engineered strain, and was denoted as the pET-DE3 engineered strain.
[0091] (1) Ind...
Embodiment 3
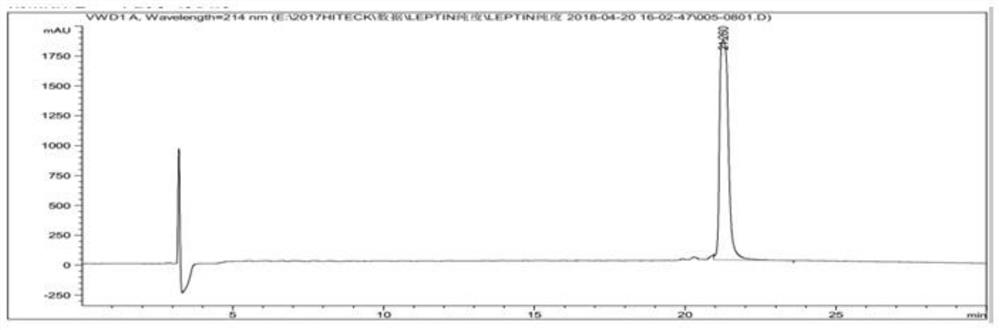
[0103] Example 3: Small-scale fermentation of pET-DE3 engineering bacteria and preparation and washing of inclusion bodies
[0104] Get the pET-DE3 engineering bacterium that freezes the embodiment 2.1 that constructs, after activation, expanded culture, inoculate in 5L fermentation medium by 10% inoculum size (fermentation medium composition is: glucose 5.0g / L, peptone 6.0g / L L, yeast powder 12.0g / L, NaCl1.0g / L, NH 4 Cl 2g / L, Na 2 HPO 4 12H 2 O 30.0g / L, KH 2 PO 4 3.0g / L, MgSO 4 ·7H 2 O 1.0g / L) in the fermentation culture (temperature 37 ℃, rotating speed 200rpm, ventilation rate 1.8Nm 3 / h, tank pressure 0.2bar); control the dissolved oxygen above 30% in the early stage of fermentation, gradually adjust the speed to 600rpm when it is insufficient, and then adjust the ventilation to 2.5Nm 3 / h, and finally fed with carbon source (glucose 600g / L) and nitrogen source (peptone 150g / L, yeast powder 150g / L) to control dissolved oxygen at about 30%; in the early stage of fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com