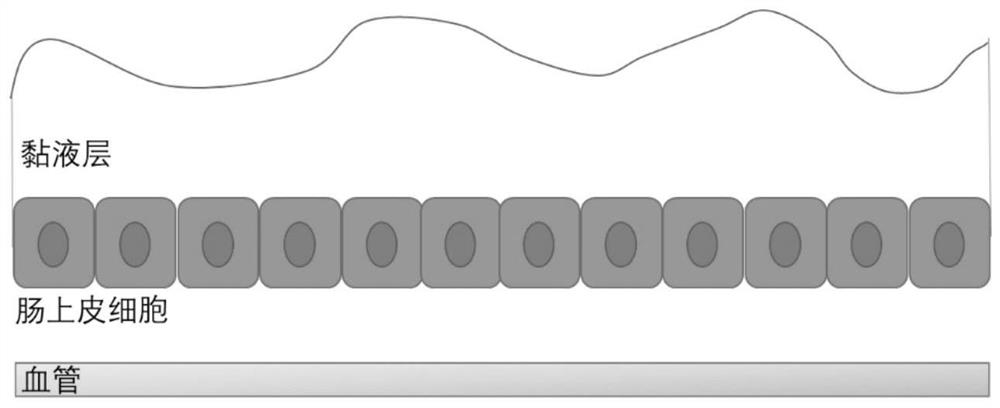
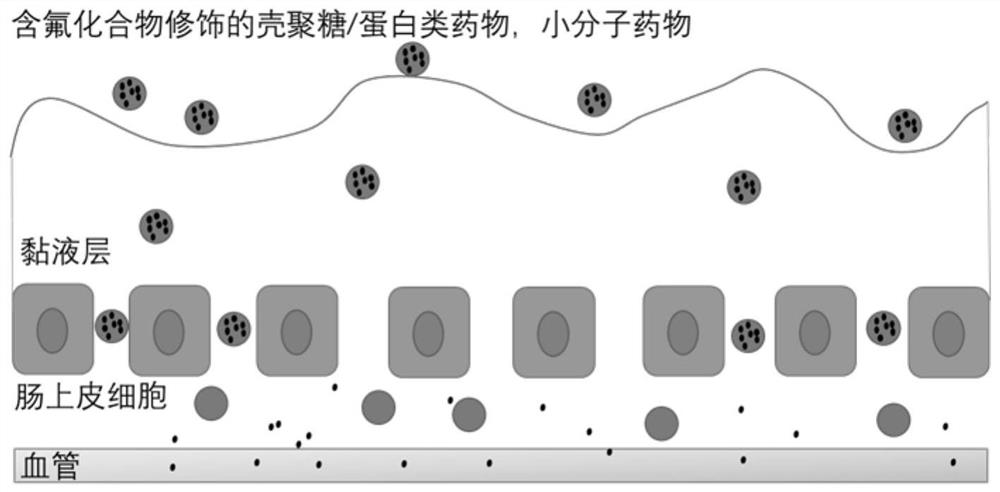
Application of fluorine-containing compound modified cationic polymer in preparation of transmucosal administration of drugs
A cationic polymer and compound technology, applied in the fields of polymer chemistry and medical biomaterials, can solve problems such as mucosal, epithelial damage, toxic and side effects of the body, and poor biocompatibility, and achieve the effects of simple operation, good effect and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1: Preparation of perfluoroheptanoic acid-modified chitosan as a carrier of oral drugs, oral delivery of insulin, diabetes treatment.
[0137] specific method:
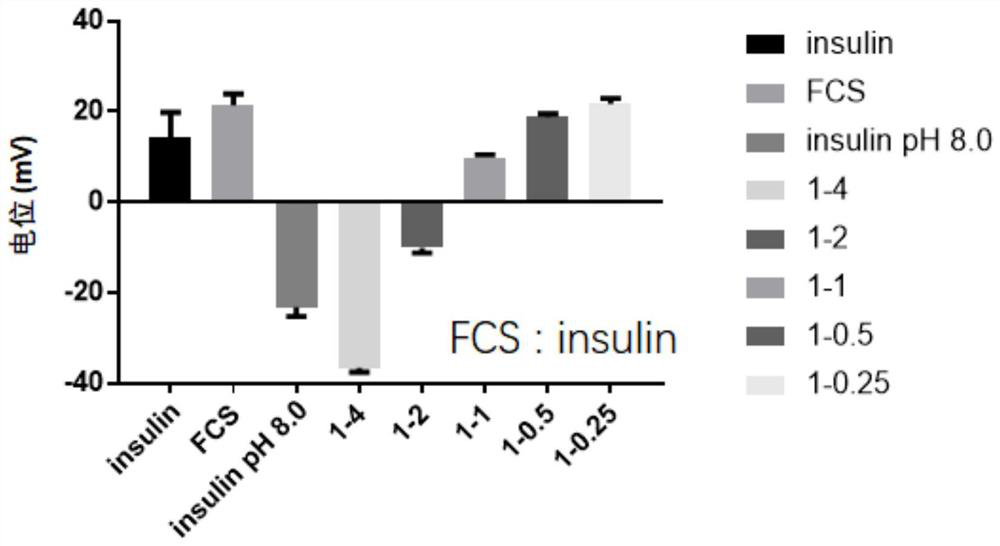
[0138] 1. Preparation of perfluoroheptanoic acid-modified chitosan-insulin capsules: Dissolve perfluoroheptanoic acid-modified chitosan and insulin in a weak acid solution to make them evenly dissolved. After mixing, add a weak base solution dropwise while stirring , adjust the pH to 6-7, and under neutral conditions, the perfluoroheptanoic acid-modified chitosan and insulin combine together due to electrostatic adsorption to form stable nanoparticles. After the reaction is complete, take it out, pre-add a freeze-drying protective agent, and freeze-dry to obtain a perfluoroheptanoic acid-modified chitosan-insulin freeze-dried powder, which is packed into a capsule and wrapped with an enteric coating.
[0139] 2. The chitosan-insulin freeze-dried powder modified by reconstituted perfluoroheptanoic acid ...
Embodiment 2
[0146] Example 2: Preparation of perfluoroheptanoic acid-modified chitosan as a carrier oral capsule, oral delivery of apoptosis-ligand 1 antibody, observation of different proportions of perfluoroheptanoic acid-modified chitosan-apoptosis-ligand Transmucosal effects of body 1 antibody particles.
[0147] specific method:
[0148] 1. Preparation of perfluoroheptanoic acid-modified chitosan-apoptosis-ligand 1 antibody particles: Weigh 0.2 mg perfluoroheptanoic acid-modified chitosan and dissolve it in 0.5 mL ultrapure water to obtain 0.4 mg / mL perfluoroheptanoic acid-modified chitosan Fluoroheptanoic acid-modified chitosan, and serially diluted to obtain 0.4mg / mL, 0.2mg / mL, 0.1mg / mL, 0.05mg / mL, 0.0025mg / mL perfluoroheptanoic acid-modified chitosan solution . Use fluorescently-labeled immunoglobulin G as a template instead of the apoptosis-ligand 1 antibody, and drop into 0.5mL of 0.1mg / mL FITC-labeled immunoglobulin G of 0.02M pH=7.2 under the condition of constant stirring ...
Embodiment 3
[0185] Example 3: Using perfluoroheptanoic acid modified chitosan as a carrier to prepare perfluoroheptanoic acid modified chitosan-immune modulator particles, administered in the form of spray / inhalation, to investigate the ability of drug pulmonary delivery .
[0186] specific method:
[0187] 1. Preparation of perfluoroheptanoic acid-modified chitosan-antibody particles: Add apoptosis-ligand 1 antibody to perfluoroheptanoic acid-modified chitosan aqueous solution, and stir at room temperature for 1 hour to form stable nanoparticles.
[0188] 2. The perfluoroheptanoic acid-modified chitosan-antibody particles were delivered in aerosol through the pulmonary administration needle, and tissue sections were performed 24 hours after delivery to observe the retention of the fluorescent signal in the lung tissue. The result is as Figure 3-1 shown.
[0189] For experimental results, see Figure 3-1 , perfluoroheptanoic acid-modified chitosan-antibody particles can stay in the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com