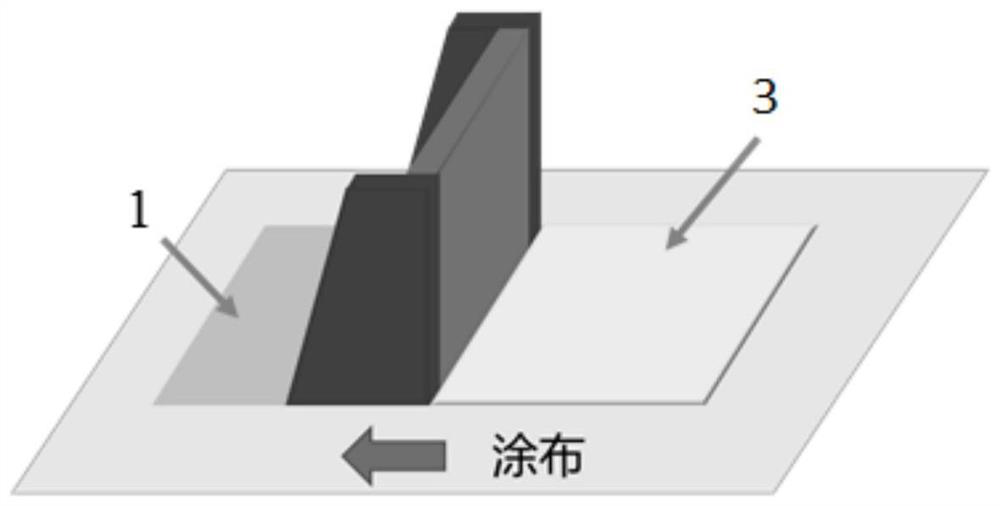
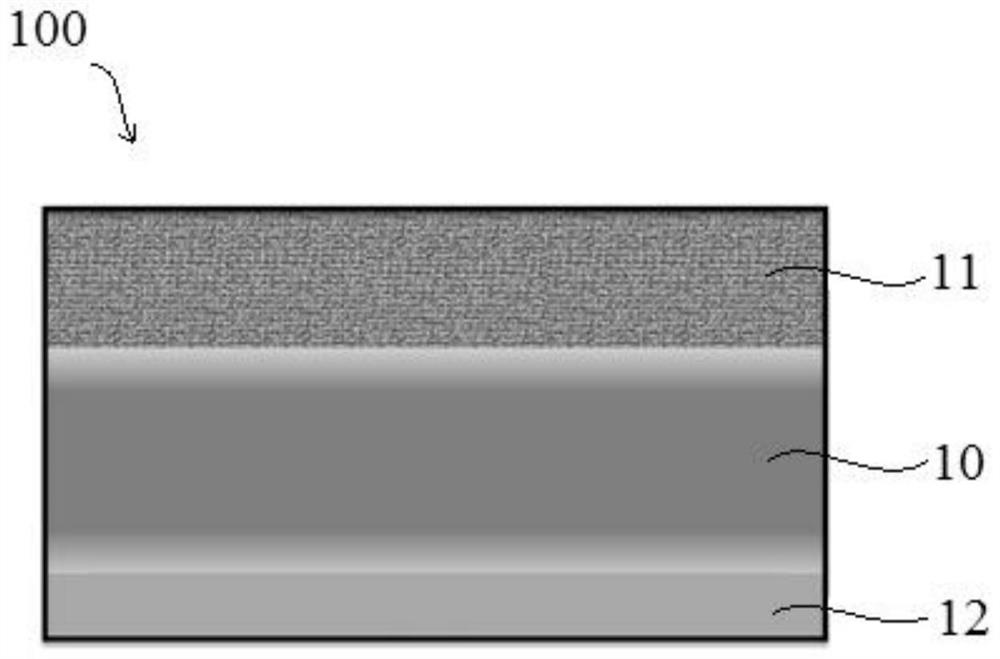
Solid composite electrolyte membrane, manufacturing method thereof and polymer secondary battery
A composite electrolyte membrane, solid polymer technology, used in secondary batteries, secondary battery repair/maintenance, electrolytes, etc., can solve the problems of incompatibility, complex process, low productivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0152] The preparation method of the positive electrode 11 may include the following steps:
[0153] Mixing process: dissolving or dispersing the positive electrode active material, conductive agent, and binder in the solvent according to a predetermined ratio, stirring or grinding them to make them evenly mixed, and preparing a slurry.
[0154] Coating process: uniformly coat the above-mentioned slurry on the current collector, and control the thickness of the slurry.
[0155] Drying process: drying the above-mentioned coated current collector in the atmosphere at 50-100°C for 2h, then transferring to a vacuum state and drying at 100-150°C for 8-24h to obtain the positive electrode.
[0156] The method for manufacturing the positive electrode has been described above, but the above-mentioned manufacturing method is also applicable to the preparation of the negative electrode material.
[0157]
[0158] In the polymer secondary battery 100 of the present invention, the nega...
Embodiment
[0168] The present invention will be further described below through examples, but the present invention is not limited by these examples and the like.
[0169] The measurement methods used in the present invention are as follows.
[0170] [Structure and purity]
[0171] For the synthesized polymers and plastic crystals, their structure and purity were confirmed by H NMR spectrum ( 1 H-NMR) were characterized. The instrument used was AVANCE III HD500 produced by Bruker, Germany, and the deuterated reagent used was deuterated dimethyl sulfoxide, deuterated dichloromethane, deuterated acetone or deuterated water.
[0172] [polymer molecular weight]
[0173] The molecular weight of the synthesized polymer was measured by gel permeation chromatography (GPC). The measurement conditions are: mobile phase: N,N-dimethylformamide, tetrahydrofuran, cyclohexane, etc., test temperature: 10-60°C, standard sample: polymethyl methacrylate, polyethylene glycol, polystyrene Wait. The ins...
Synthetic example 1
[0189] Synthesis of polyacrylonitrile
[0190]Under an argon atmosphere, 40 mL of toluene was added to a dry 150 mL three-necked flask, stirred and heated to 80° C., and kept for 15 min. 5.53 g (0.1 mol) of acrylonitrile and 0.03 g of azobisisobutyronitrile as a polymerization initiator were added dropwise through a constant pressure dropping funnel, and reacted for 7 hours. Add hydrochloric acid / methanol solution (volume ratio V of hydrochloric acid and methanol 盐酸 :V 甲醇 =1:10) 10 mL to terminate the reaction. After the obtained solid was filtered, it was washed twice with 20ml of acetone, twice with 20ml of deionized water, twice with 20ml of acetone, and then dried in a vacuum oven at 80°C for 24 hours to obtain a pale yellow acetone insoluble polymer. Acrylonitrile powder.
[0191] The structure of the resulting product 1 H-NMR was determined, the resulting 1 H-NMR picture as Figure 4 shown. The deuterated reagent used was deuterated dimethyl sulfoxide.
[0192]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com