Synthetic method of high-purity dopamine hydrochloride
A technology of dopamine hydrochloride and a synthesis method, applied in the field of medicinal chemistry, can solve the problems of many by-products, difficult to realize industrial production, difficult to purify, etc., and achieves the effects of less three wastes and simple post-processing method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
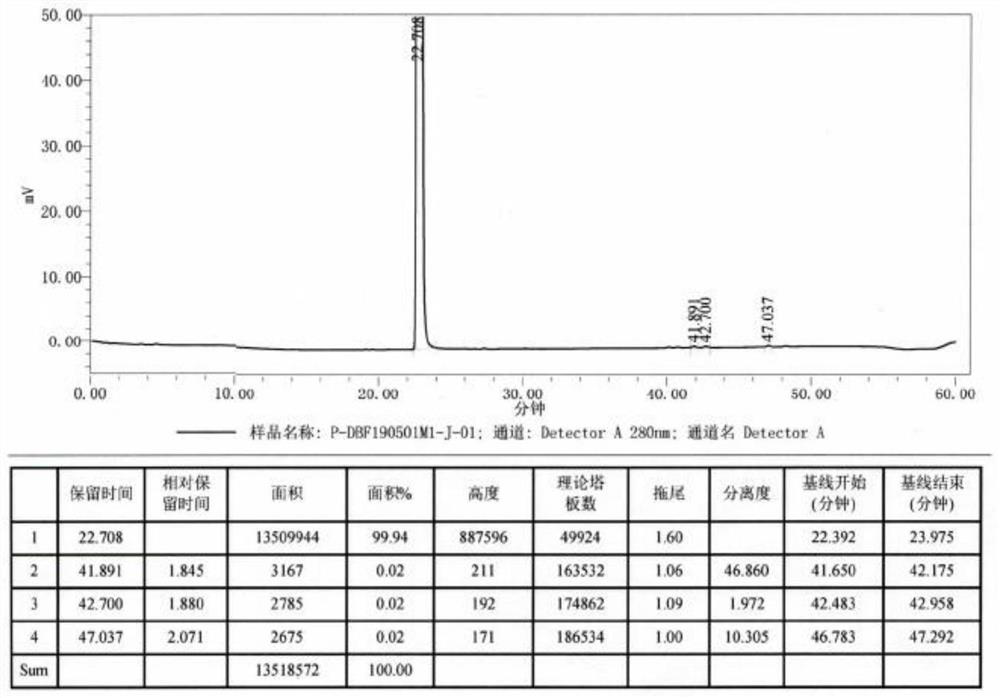
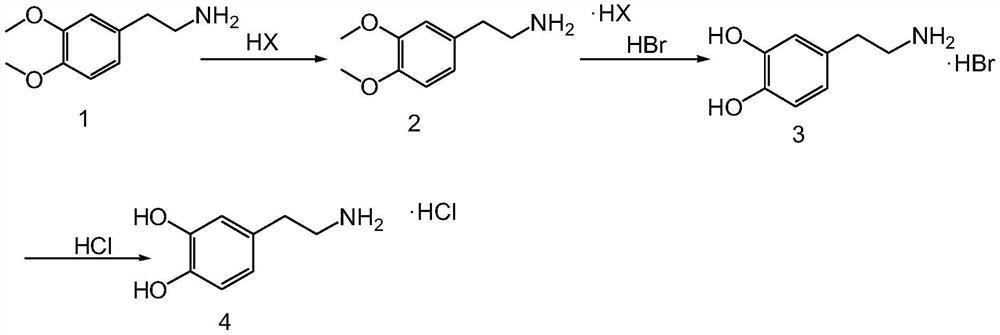
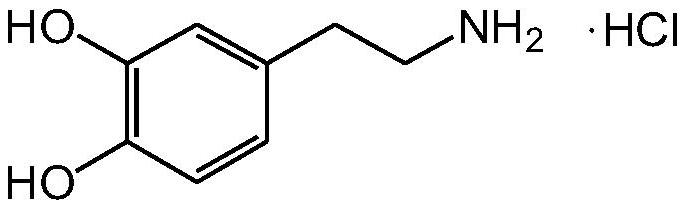
Image
Examples
Embodiment 1
[0035] Mix 3.4-dimethoxyphenethylamine (200.0g, 1.10mol) with 800ml of ethanol, add dropwise 204.0g (1.21mol) of hydrobromic acid with a concentration of 48%, control the internal temperature not higher than 50°C, add dropwise After completion, the reaction was stirred for 0.5 hours. Stir and cool down to 20-30°C for crystallization for 2 hours, filter to obtain the crude product of 3,4-dimethoxyphenethylamine hydrobromide, recrystallize the wet product with absolute ethanol, filter, and air-dry to obtain a white powder Solid 3.4-dimethoxyphenethylamine hydrobromide 196.7g, purity 99.84%, yield 68.2%;
[0036] Mix 196.7 g of 3.4-dimethoxyphenethylamine hydrobromide with 1573.6 g of 48% hydrobromic acid, replace with nitrogen, and heat to an internal temperature of 110-115° C. for 5 hours. Turn off the heating, stir and cool down to 20-30°C for crystallization for 2 hours, filter, and vacuum-dry to obtain 146.0 g of white solid dopamine hydrobromide with a purity of 99.92% and...
example 2
[0039] Mix 3.4-dimethoxyphenethylamine (200.0 g, 1.10 mol) with 600 ml of isopropanol, add hydrochloric acid (223.0 g, 2.20 mol) dropwise in a water bath to control the internal temperature not higher than 50 °C, and dropwise is completed. Evaporate the solvent, add 600ml of ethyl acetate, stir and crystallize, filter to obtain a light yellow solid, add absolute ethanol to recrystallize, filter, and blow dry to obtain 170.5g of white powdery solid 3.4-dimethoxyphenethylamine hydrochloride , purity 99.81%, yield 71.2%;
[0040] Mix 170.5 g of 3.4-dimethoxyphenethylamine hydrochloride with 1023.0 g of 40% hydrobromic acid, replace with nitrogen, and heat to an internal temperature of 100-110° C. for 5 hours. Turn off the heating, stir and cool down to 20-30°C for crystallization for 2 hours, filter, and vacuum-dry to obtain 139.5 g of white solid dopamine hydrobromide with a purity of 99.83% and a yield of 76.1%;
[0041] Mix 139.5 g of dopamine hydrobromide with 1116 ml of abs...
example 3
[0043]Mix 3.4-dimethoxyphenethylamine (200.0g, 1.10mol) with 800ml of isopropanol, add dropwise 48% hydrobromic acid (222.5g, 1.32mol) to control the internal temperature not higher than 50°C, and dropwise , stirred and cooled to 20-30 ° C for 2 hours, filtered to obtain a light yellow solid, added absolute ethanol to recrystallize, filtered, and air-dried to obtain a white powdery solid 3.4-dimethoxyphenethylamine hydrobromide 192.3 g, purity 99.82%, yield 66.7%;
[0044] Mix 192.3 g of 3.4-dimethoxyphenethylamine hydrobromide with 961.5 g of 48% hydrobromic acid, replace with nitrogen, and heat to an internal temperature of 110-120° C. for 5 hours. Turn off the heating, stir and cool down to 20-30°C for crystallization for 2 hours, filter, and vacuum-dry to obtain 136.2 g of white solid dopamine hydrobromide with a purity of 99.85% and a yield of 79.3%;
[0045] Mix 136.2 g of dopamine hydrobromide with 1,089.6 ml of isopropanol, replace with nitrogen, heat up to 80° C. and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com