Feline panleukopenia virus antibody sequence, tetrapeptide chain molecule, globulin molecule and application
A feline parvovirus and sequence technology, applied in the fields of feline parvovirus antibody sequence, immunoglobulin molecule and its application, and tetrapeptide chain molecule
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
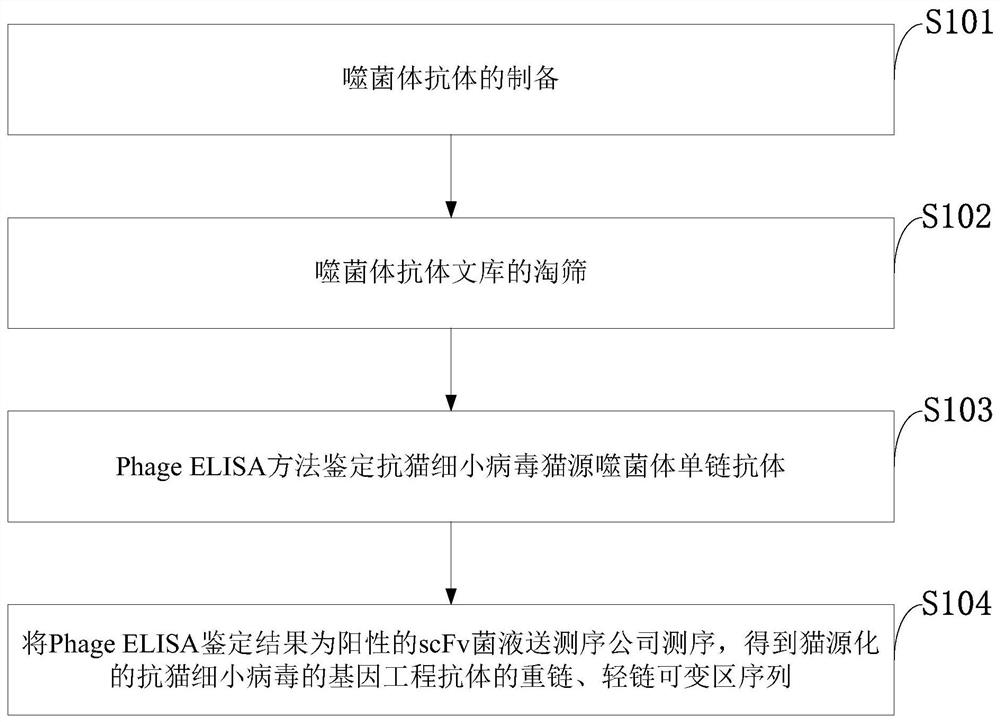
[0062] Example 1 Construction of cat-derived anti-feline parvovirus single-chain antibody library
[0063] 1. Isolation of Feline Peripheral Blood Lymphocytes
[0064] According to the International Animal Immunization Manual, 5 experimental cats were immunized with vaccine. Each immunization interval was 2 weeks, and a total of 3 immunizations were performed. Two weeks after the three immunizations, the peripheral blood of the cats was collected. Take 5-10mL of fresh blood and mix evenly with whole blood and tissue diluent at a ratio of 1:1-1:2. Add cell separation solution to a 15mL centrifuge tube, and gently add an equal volume of diluted anticoagulant blood along the tube wall. The horizontal centrifuge is centrifuged at a speed of 400g-800g and a time of 15min-25min. After centrifugation, the liquid in the centrifuge tube should be divided into four layers, from top to bottom: the first layer: plasma layer; the second layer: lymphocyte layer; The third layer: separat...
Embodiment 2
[0071] Example 2 Screening of cat-derived anti-feline parvovirus single-chain antibody library
[0072] 1. Preparation of Phage Antibody
[0073] Add 1 mL of phage primary antibody library to 3 mL of XLI-Blue bacterial solution with OD600=0.5, place at 37°C for 45 min, add 6 mL of LB liquid medium containing Amp+ (100 μg / mL), and add glucose (1mol / mL) at a ratio of 1:1000 L) Continue to cultivate, when the OD600 of the bacterial solution is 0.5, add the helper phage M13K07, place it in a 37°C incubator for 30min, and then incubate at 220rpm / min at 37°C for 1h, centrifuge the bacterial solution at 3500rpm for 10min, discard the supernatant, and use an equal volume LB liquid medium containing Amp+ (100 μg / mL) and Kana (50 μg / mL) was added with IPTG (1 mol / L) at a ratio of 1:1000, 220 rpm / min, 37°C, and shaken for 6 hours. Centrifuge the culture medium at 12000rpm for 10-20min, and collect the supernatant. Add PEG8000 at a ratio of 1:4-1:5, invert and mix well, at this time clo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com