A kind of method for preparing isobutyryl thiazolyl resorcinol
A technology based on thiazolyl resorcinol and isobutyramide, which is applied in the field of chemical synthesis, can solve the problems of unfriendly environment, many side reactions, and high price, and achieve simple synthesis process route, convenient post-treatment, and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
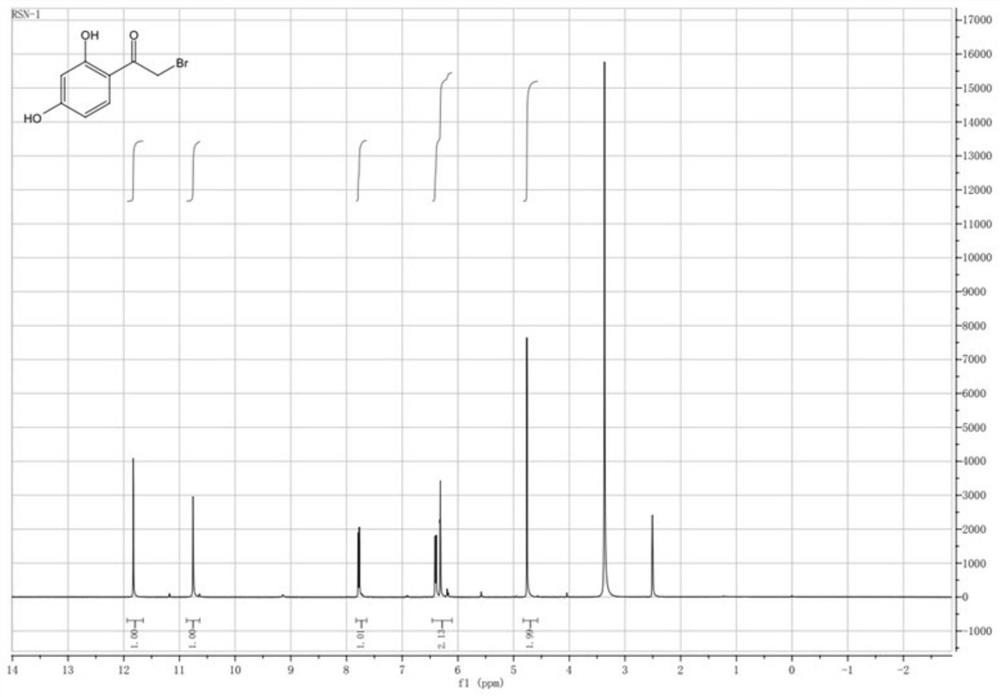
[0032] Example 1: Synthesis of Intermediate I
[0033] Weigh 2220 grams of resorcinol, 2100 grams of bromoacetic acid, and 5500 mL of 48% boron trifluoride ether solution, respectively, and add them to a 10-liter reaction flask, and slowly heat to 75 ° C (first heat to 65 ° C for 30 min, then heat up to 75 ° C.) °C to continue the reaction), the tail gas is absorbed with an aqueous solution of sodium hydroxide. After the reaction reaches 3 hours, the content of bromoacetic acid is tracked in the liquid phase, and after less than 1%, cooled to room temperature, slowly added dropwise with 3000 ml of ice water, stirred for 10 minutes, liquid separation, dried organic phase, and concentrated organic phase to obtain about 2900 grams of oily matter . The oily substance was separated by petroleum ether:ethyl acetate=50:1 column chromatography to obtain about 1800 g of off-white to light yellow solid, the purity was more than 98%, and the yield was 51%.
Embodiment 2
[0034] Example 2: Synthesis of Intermediate II
[0035] Weigh 1,000 grams of isobutyryl chloride, 893 grams of thiourea, and 3 L of toluene, respectively, add them to a 5-liter reaction flask, perform a reflux reaction at 115°C for 3-4 hours, follow the liquid phase, and absorb the tail gas with an aqueous solution of sodium hydroxide. 3 liters of water was added, and after toluene was distilled, the mixture was cooled and crystallized. About 900 grams of yellow crystalline solid were obtained by filtration, the purity was more than 98%, and the yield was about 65%.
Embodiment 3
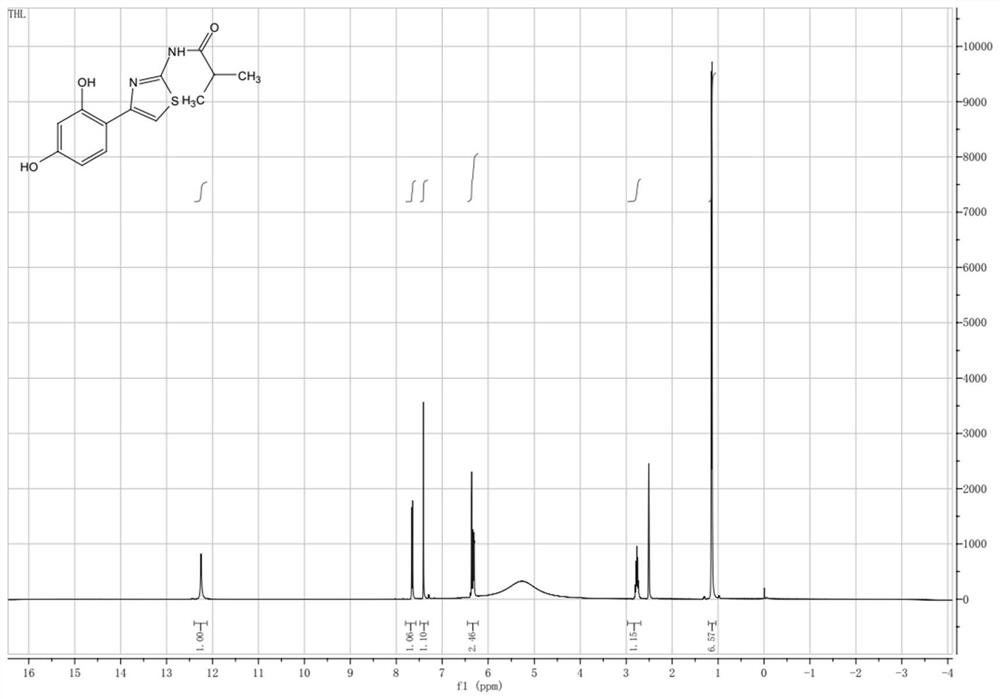
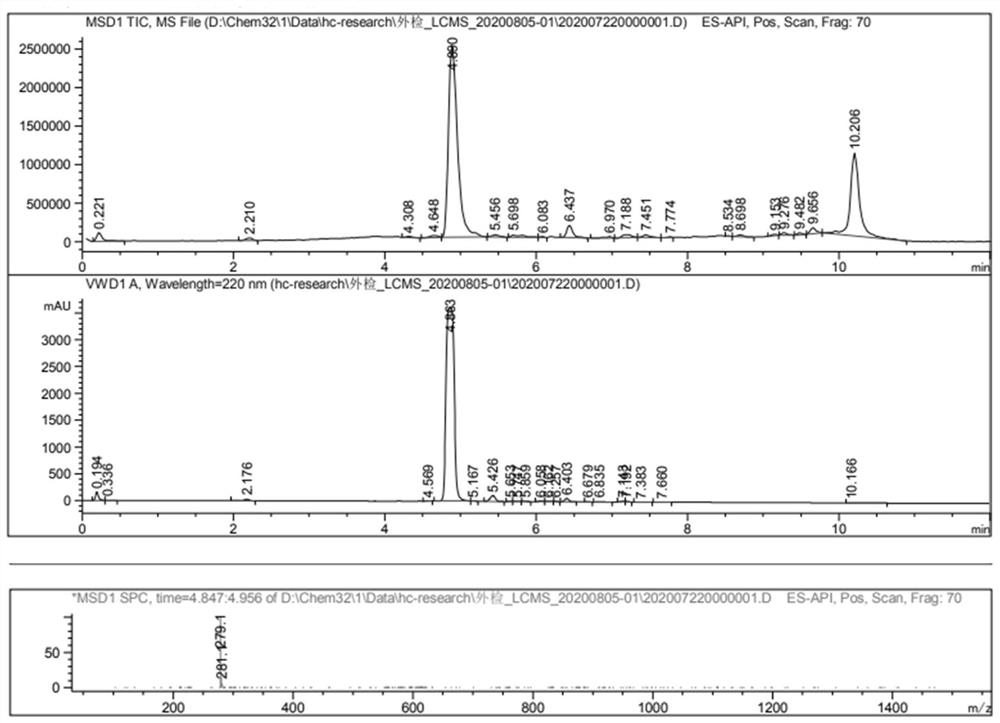
[0036] Example 3: Synthesis of Thiamidol
[0037] Weigh 500 grams of Intermediate I, 333 grams of Intermediate II, 2.5 L of ethanol, and 285 grams of sodium bicarbonate, respectively, add them to a 5-liter reaction flask, and slowly heat up to 80 ° C. After 30 minutes of reaction, the liquid phase is tracked, and the reaction is completed. After that, filter the inorganic salt, concentrate the organic matter, 1.5L ethyl acetate hot slurry, after cooling, filter the product to obtain 530 grams of white to off-white solid, the purity is more than 99%, and the yield is about 88%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com