Polymer prodrug micelle with reduction responsiveness as well as preparation method and application thereof
A polymer and responsive technology, applied in the field of biomedicine, can solve the problems of different drug metabolism rates, cumbersome preparation methods, obvious toxic and side effects, etc., and achieve the effects of fast and simple preparation process, improved reaction efficiency, and improved inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] Following 7-7,-dithiodiheptanoic acid can be synthesized according to prior art, and its preparation method is provided below, comprising the following steps:
[0057] 1) Cycloheptanolactone is prepared from cycloheptanone;
[0058] Put the compound m-chloroperoxybenzoic acid (335mmol) into the reaction flask, add dichloromethane (500mL) and cycloheptanone (223mmol) successively at 0°C, and react for 7 days; (300mL×2) was washed twice, the organic layer was washed twice with saturated sodium bicarbonate solution (300mL×2); the organic layer was dried with anhydrous sodium sulfate, suction filtered and spin-dried to obtain a yellow oil;
[0059] 1 H NMR (400MHz, CDCl 3 )δ=4.32–4.24(t,2H),2.52–2.44(t,2H),1.83–1.70(m,4H),1.60–1.46(m,4H). 13 C NMR (101MHz, CDCl 3 )δ=176.69, 67.87, 31.25, 30.86, 28.33, 25.77, 23.89.
[0060] 2) Preparation of 7-hydroxyheptanoic acid methyl ester by ring-opening cycloheptanolide;
Embodiment 1
[0082] A Reduction Responsive Polymer Prodrug mPEG 2000 -mMAP 12 -mPEG 2000 The preparation method of micelle, comprises the following steps:
[0083]
[0084] Preparation of S1, 1H-imidazole-1-carboxylic acid-N-tert-butoxycarbonyl-3-aminobenzyl ester;
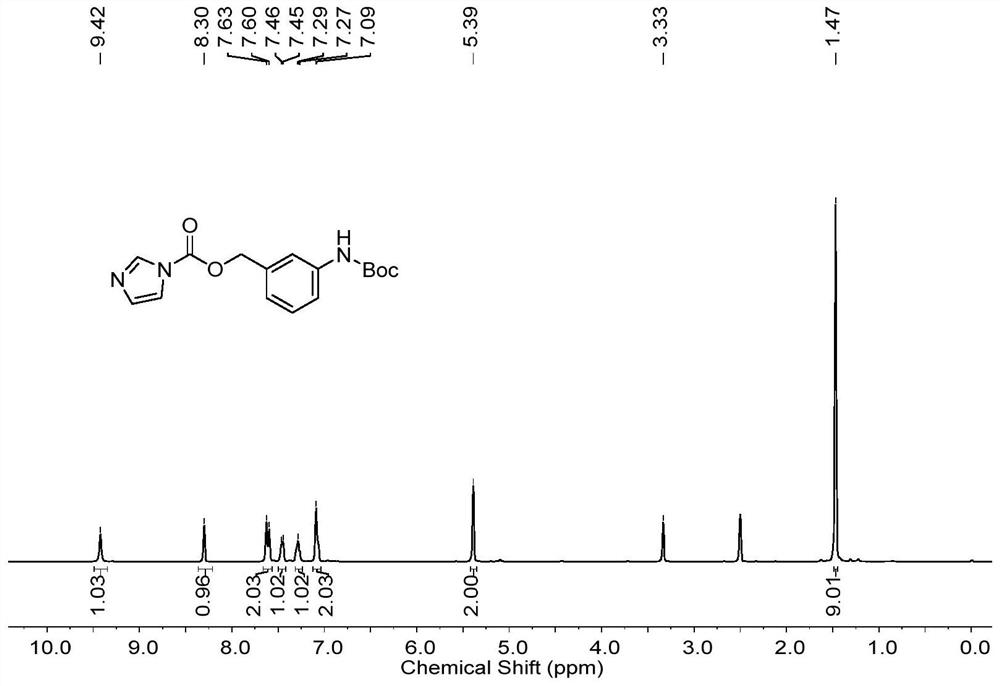
[0085] Dissolve N-tert-butoxycarbonyl-3-aminobenzyl alcohol (1.5mmol) in tetrahydrofuran (1.5mL), add dropwise carbonyldiimidazole (5mmol) dissolved in tetrahydrofuran (4.5mL), react at 25°C for 12h; spin dry Add 10 mL of ethyl acetate to dissolve, wash with saturated brine (10 mL×2) twice; dry the organic phase over anhydrous sodium sulfate, filter with suction and spin dry to obtain a yellow oil; figure 1 gives the resulting product 1 H NMR spectrum.
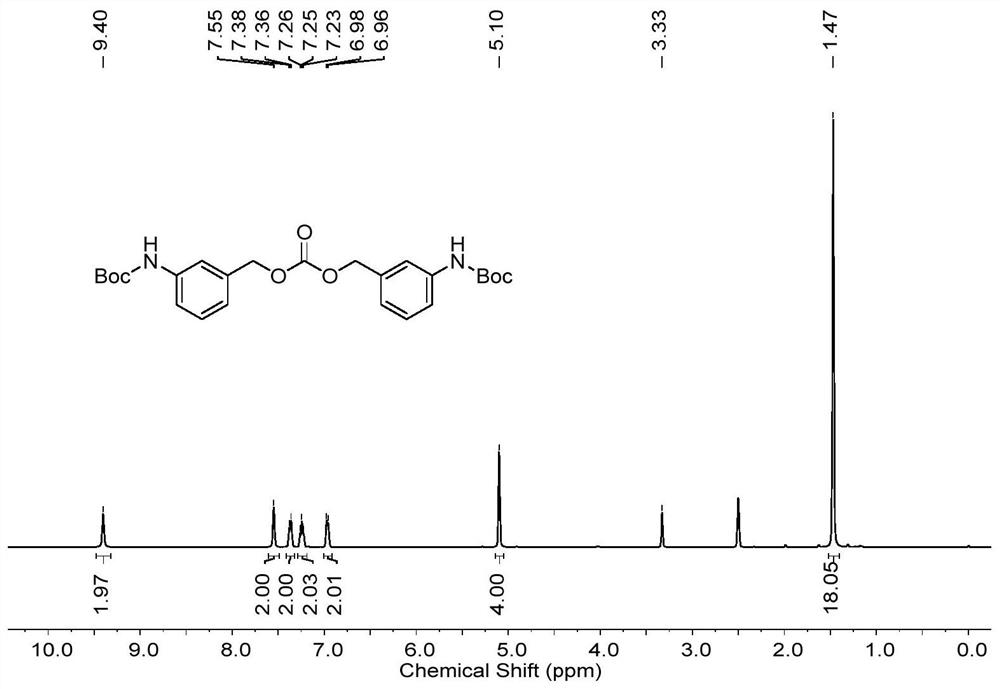
[0086] S2, preparation of bis-N-tert-butoxy-3-aminobenzyl carbonate;
[0087] Add the compound N-tert-butoxycarbonyl-3-aminobenzyl alcohol (35mmol) into a three-necked flask, add tetrahydrofuran (70mL), add sodium hydride (35mmol) at 0°C and stir for 1h, then drop int...
Embodiment 2
[0097] A Reduction Responsive Polymer Prodrug mPEG 2000 -mMAP 26 -mPEG 2000 The preparation method of micelle, step is with embodiment 1, difference is:
[0098] S1, react at 20°C for 20 hours, the molar ratio of N-tert-butoxycarbonyl-3-aminobenzyl alcohol to carbonyldiimidazole is 1:2;
[0099] S2. Reaction at 20°C for 10 hours, 1H-imidazole-1-carboxylic acid-N-tert-butoxycarbonyl-3-aminobenzyl ester:N-tert-butoxycarbonyl-3-aminobenzyl alcohol:sodium hydride is 1:0.5:0.5;
[0100] S4, the reaction time is set to 10min;
[0101] S5, the HOOC-mMAP 12 -COOH is replaced by HOOC-mMAP 26 -COOH, reacted at 20℃ for 24h, HOOC-mMAP 26 -COOH:mPEG-NH 2 :HOAT:HATU is 1:2:3:3;
[0102] S6, the mPEG 2000 -mMAP 12 -mPEG 2000 Replace with mPEG 2000 -mMAP 26 -mPEG 2000 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com