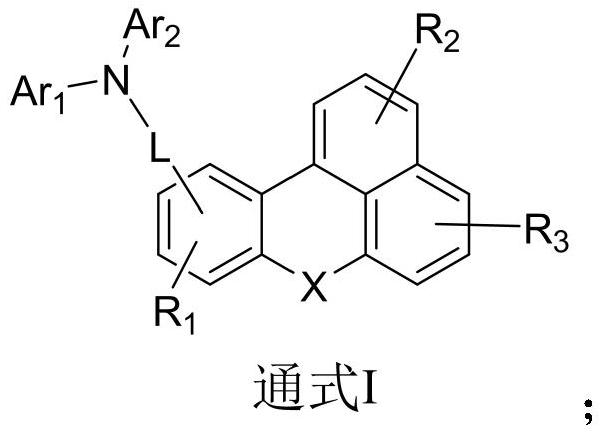
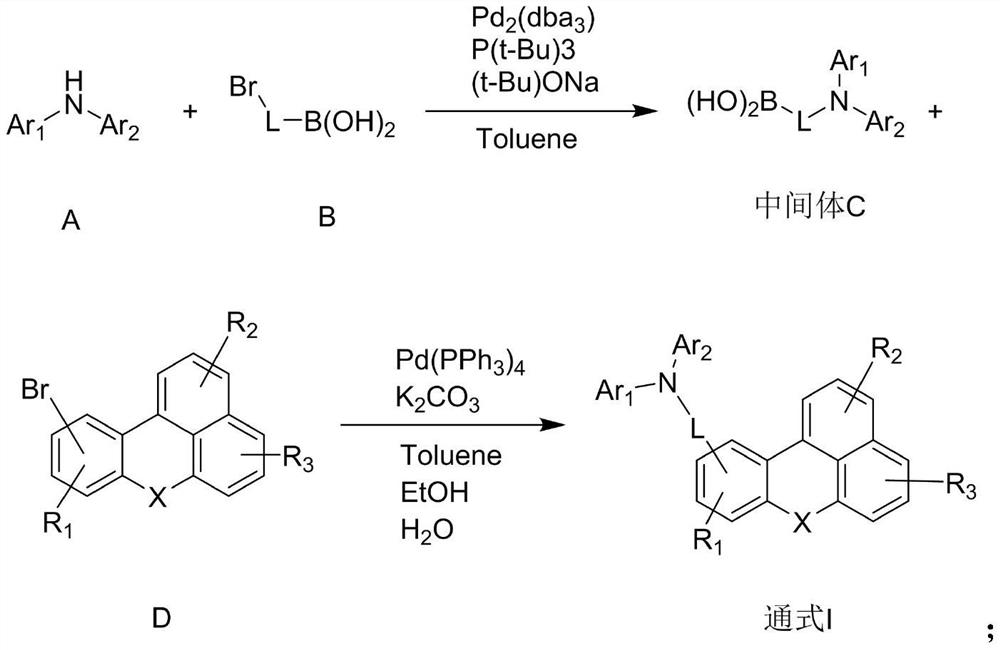
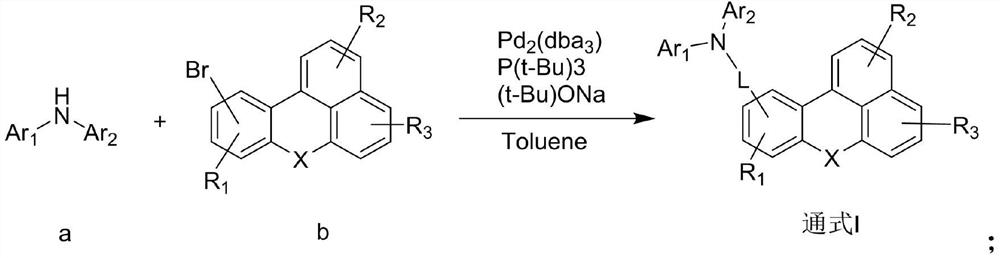
Organic electroluminescent compound of benzanthracene derivative and preparation method and application thereof
An electroluminescent device and derivative technology, which is applied in the preparation of organic compounds, silicon organic compounds, aminohydroxy compounds, etc. Level transition smooth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the preparation of compound 9
[0056]
[0057] After adding reactant A-9 (50mmol) and reactant B-9 (55mmol) in 200ml of toluene in the reaction vessel, add Pd under nitrogen atmosphere 2 (dba) 3 (0.56mmol), P(t-Bu) 3 (2.8 mmol), t-BuONa (150 mmol). After the addition, the reaction temperature was slowly raised to 110 °C, and the mixture was stirred for 10 h. Use diatomaceous earth to filter while hot to remove salt and catalyst. After the filtrate is cooled to room temperature, distilled water is added to the filtrate for washing. After liquid separation, the organic phase is retained, and the aqueous phase is extracted with ethyl acetate. The combined organic layers were then dried using magnesium sulfate, and the solvent was removed using a rotary evaporator. Using dichloromethane:petroleum ether at a volume ratio of 1:9 as the eluent, the remaining substance was purified by column chromatography to obtain the final product 9 (21.7 g, yield 78%, ...
Embodiment 2
[0058] Embodiment 2: the preparation of compound 23
[0059]
[0060] step 1:
[0061] After adding chemical formula reactant A-23 (50mmol) and reactant B-23 (55mmol) in 200ml toluene in the reaction vessel, add Pd under nitrogen atmosphere 2 (dba) 3 (0.56mmol), P(t-Bu) 3 (2.8 mmol), t-BuONa (150 mmol). After the addition, the reaction temperature was slowly raised to 110 °C, and the mixture was stirred for 10 h. Use diatomaceous earth to filter while hot to remove salt and catalyst. After the filtrate is cooled to room temperature, distilled water is added to the filtrate for washing. After liquid separation, the organic phase is retained, and the aqueous phase is extracted with ethyl acetate. The combined organic layers were then dried using magnesium sulfate, and the solvent was removed using a rotary evaporator. Using dichloromethane:petroleum ether volume ratio: 9 as eluent, the remaining substance was purified by column chromatography to obtain intermediate C-23 ...
Embodiment 3
[0064] Embodiment 3: the preparation of compound 44
[0065]
[0066] step 1:
[0067] After adding chemical formula reactant A-44 (50mmol) and reactant B-44 (55mmol) in 200ml toluene in the reaction vessel, add Pd under nitrogen atmosphere 2 (dba) 3 (0.56mmol), P(t-Bu) 3 (2.8 mmol), t-BuONa (150 mmol). After the addition, the reaction temperature was slowly raised to 110 °C, and the mixture was stirred for 10 h. Use diatomaceous earth to filter while hot to remove salt and catalyst. After the filtrate is cooled to room temperature, distilled water is added to the filtrate for washing. After liquid separation, the organic phase is retained, and the aqueous phase is extracted with ethyl acetate. The combined organic layers were then dried using magnesium sulfate, and the solvent was removed using a rotary evaporator. Using dichloromethane:petroleum ether at a volume ratio of 1:9 as the eluent, the remaining substance was purified by column chromatography to obtain inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com