Preparation method and application of array sensor for instantaneously identifying drug-induced HK-2 cell damage
An array sensor and cell damage technology, applied in the field of biosensing, can solve the problems of cumbersome time-consuming, low sensitivity, huge equipment, etc., and achieve the effects of low cost, wide application range and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
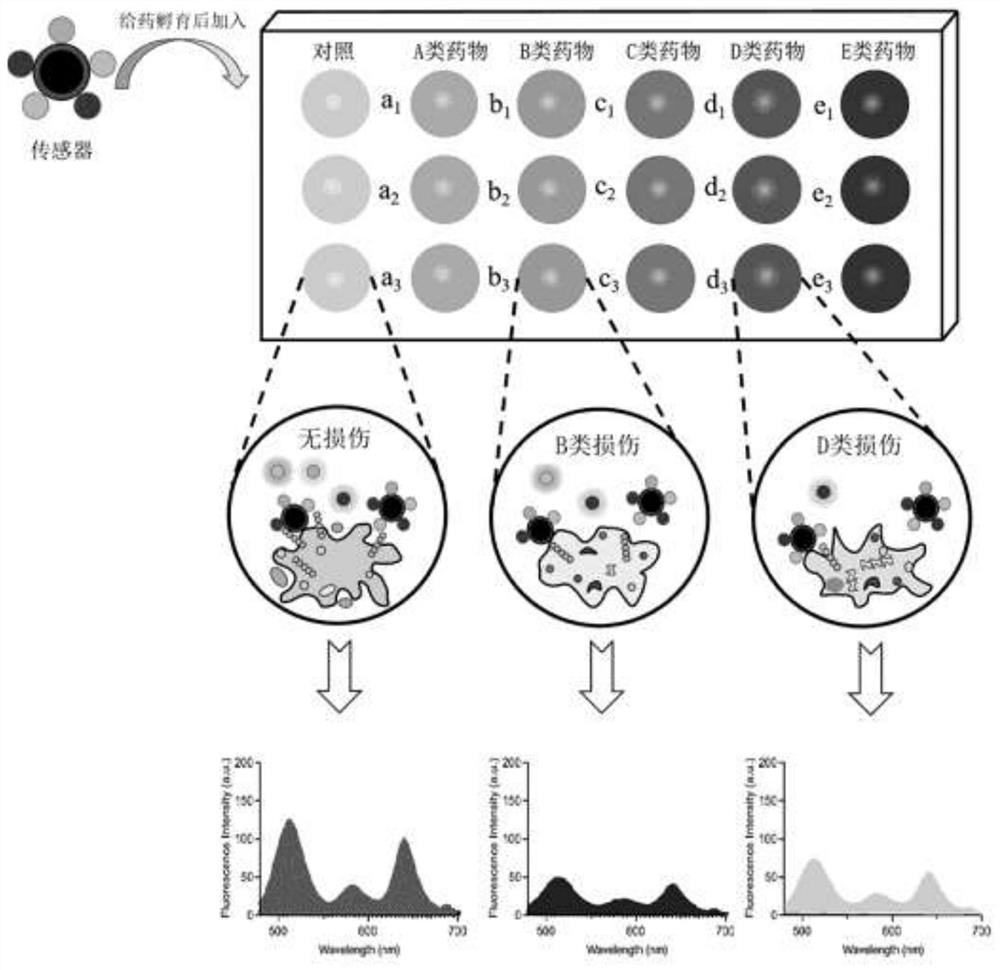
[0036] Such as figure 1 The preparation method of an array sensor that transiently recognizes drug-induced HK-2 cell damage, the specific steps are as follows:
[0037] Step (1.1), preparation of polydopamine-polyethyleneimine (PDA-PEI) copolymer carrier: Add dopamine hydrochloride and polyethyleneimine to Tris-HCl buffer, and stir at room temperature 10-30°C in the dark After 2-8 hours, filter and place in a dialysis bag for dialysis for 24-36 hours; remove unreacted dopamine hydrochloride and polyethyleneimine to obtain a PDA-PEI copolymer carrier;
[0038] Specifically, 1. Dilute 100 μL Tris-HCl (1M, pH=7.4) buffer solution with ultrapure water to 10 mL for later use;
[0039] 2. Weigh 10mg of dopamine hydrochloride (DA·HCl) and 10mg of polyethyleneimine (PEI, M.W.600Da) respectively, add the two into 10mL Tris-HCl buffer solution, and place on a magnetic stirrer at room temperature 10-30°C to avoid Light stirring for 2-8h;
[0040] 3. Filter the above solution with a 0....
Embodiment 1
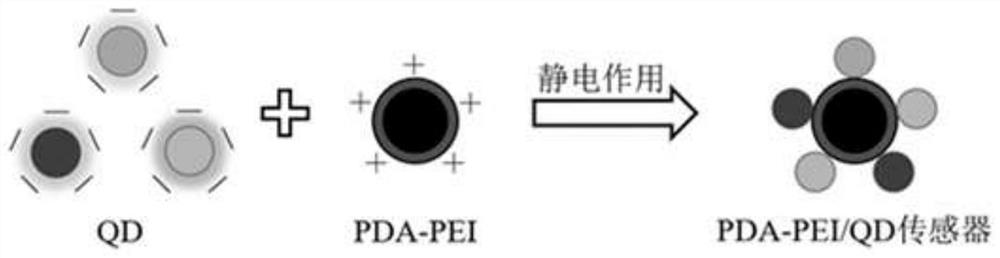
[0058] Construction of PDA-PEI / QDs sensor and verification of successful synthesis
[0059] 1. Preparation of PDA-PEI carrier
[0060] (1) Dilute 100 μL of Tris-HCl (1M, pH=7.4) buffer solution with ultrapure water to 10 mL for later use;
[0061] (2) Weigh 10mg of dopamine hydrochloride (DA·HCl) and 10mg of polyethyleneimine (PEI, M.W.600Da) respectively, add the two to 10mL Tris-HCl buffer solution, and place on a magnetic stirrer at room temperature 10-30°C Stir in the dark for 2-8 hours;
[0062] (3), filter the above solution with a 0.22 μm cellulose ester membrane, then place it in a dialysis bag (molecular weight cut-off 1000Da) and dialyze for 24-36h to remove unreacted DA and PEI to obtain polydopamine-polyethyleneimine (PDA-PEI ) copolymer carrier;
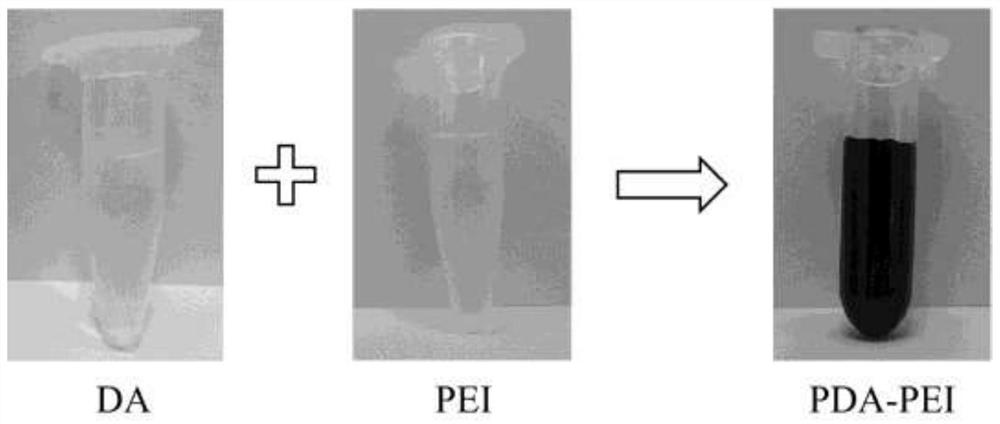
[0063] During the preparation of PDA-PEI, the successful preparation of the PDA-PEI carrier was verified by the color changes before and after the reaction, transmission electron microscope images, ultraviolet spectra...
Embodiment 2
[0078] Administration (taking isoniazid as an example), the IC is obtained by the classic MTT method 50 value:
[0079] 1. The preparation process of the sensor is consistent with Example 1;
[0080] 2. To study the cytotoxicity of isoniazid on HK-2 cells by MTT method. Include the following steps:
[0081] (1), cell plate: HK-2 cells in 4 × 10 per well 3 Density inoculation into 96-well plates, in which 200 μL of NEAA-containing MEM basal medium with a volume fraction of 12.5% FBS was added to each well (edge wells were filled with sterile PBS at pH = 7.4), at 37°C, 5% CO 2 Cultivate in the incubator for 24h;
[0082] (2), administration: After aspirating the culture solution, add 200 μ L of isoniazid solutions (without containing FBS (dissolved in MEM basal medium) was incubated at 37°C for 24 hours, and the cell morphology was observed under an inverted microscope;
[0083] (3) MTT administration: after the incubation is completed and the culture medium is sucked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com