Application of non-conjugated fluorescent alternating copolymer to preparation of fluorescent materials
A technology of alternating copolymers and fluorescent materials, which is applied in the application field of fluorescent alternating copolymers in the preparation of fluorescent materials, can solve the problems of high development costs and low quantum efficiency of photoluminescent polymers, and achieve excellent light transmittance and Thermal stability, simple synthesis method and easy scale-up, and controllable polymerization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Synthesis of the ligand: under a nitrogen atmosphere, add diacetyl (0.87g, 10.0mmol) and 3,4,5-trimethoxyaniline (5.50g, 30.0mmol) into a round bottom flask, and add toluene as a solvent and Catalytic amount of p-toluenesulfonic acid was separated from water and refluxed overnight. After the reaction was completed, the solvent was removed by rotary evaporation, and the remaining solid was recrystallized with ethanol to obtain yellow crystals with a yield of 93.0%.
[0074] 1 H NMR (400MHz, CDCl3), δ (ppm): 6.02 (s, 4H, Ar-H), 3.85 (s, 18H, OCH 3 ),2.20(s,6H,CH 3 ). 13 C NMR (100MHz, CDCl 3 ), δ(ppm): 168.70, 153.04, 147.02, 134.44, 96.03, 61.02, 56.08, 15.61.
[0075] Synthesis of α-diiminemethylpalladium chloride complex (reaction equation below): α-diiminemethylpalladium chloride complex is obtained by reacting α-diimine ligand with Pd(COD)MeCl. Under a nitrogen atmosphere, α-diimine ligand (1.1mmol) and Pd(COD)MeCl (1.0mmol) were added to a Schlenk bottle that ...
Embodiment 2
[0083] (1) The 50mL round-bottomed Schlenk bottle was continuously evacuated and baked under an infrared lamp for 3h, cooled to room temperature, replaced with CO for 3 times, and then inflated to normal pressure. Add p-benzoquinone, α-diimine palladium catalyst, solvent dichloromethane in turn, and then add styrene to start polymerization. After copolymerization at 15° C. for 6 h, the reaction solution was poured into methanol solution acidified with hydrochloric acid to terminate, and filtered to obtain 0.30 g of styrene / carbon monoxide alternating copolymer product. The molecular weight of the obtained polymer product is M n =24.6kg / mol, the dispersion coefficient is PDI=1.08, the glass transition temperature is 111°C, and the decomposition temperature is higher than 382°C.
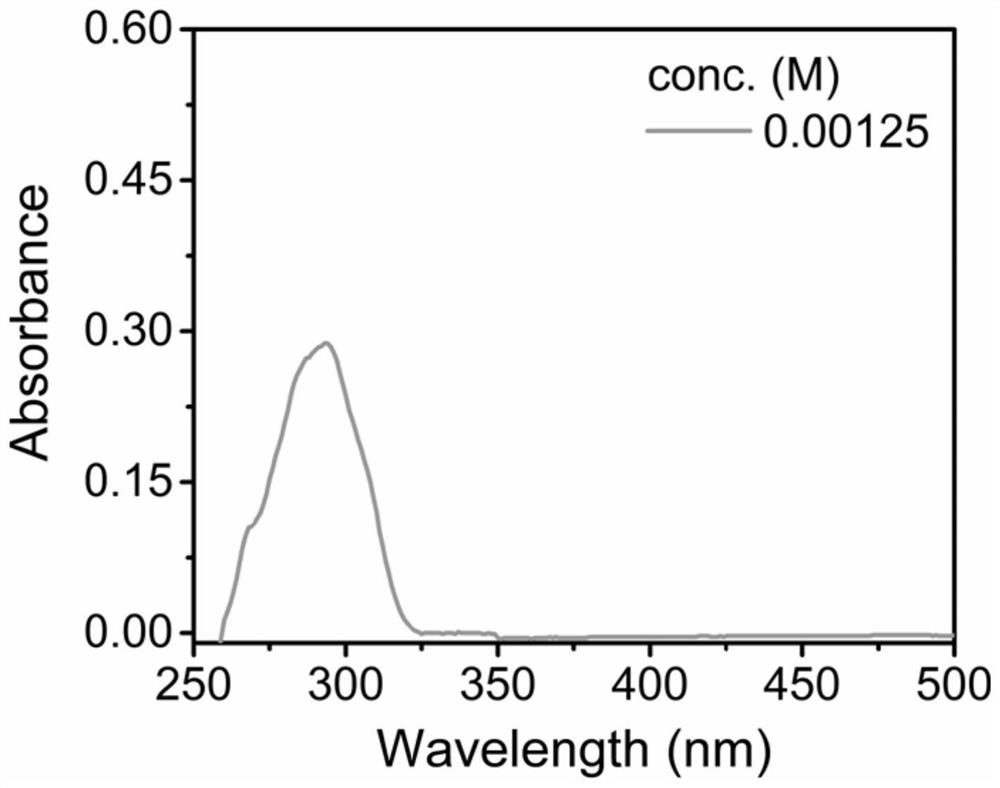
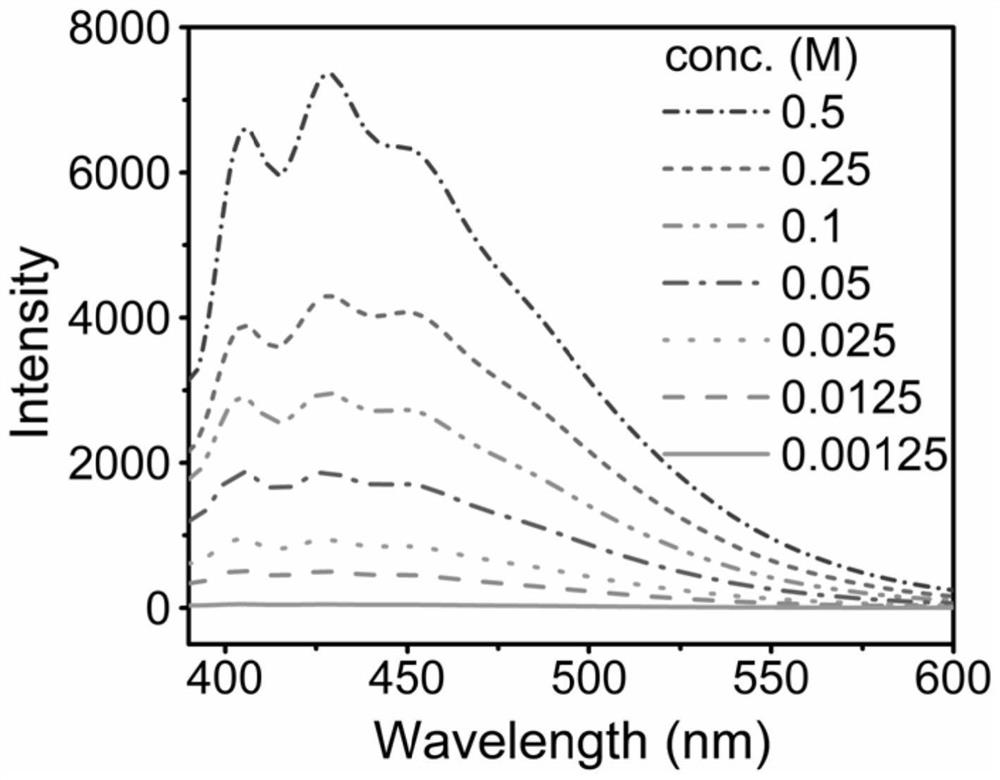
[0084](2) Dissolve 1.675 mg of the styrene / carbon monoxide alternating copolymer obtained in step (1) in 10 mL of tetrahydrofuran (it should be noted that in this system, 1 mol / L=134 mg / mL, so 0.1675 ...
Embodiment 3
[0089] (1) The polymerization condition identical with embodiment 2, copolymerization time is 12 hours. 0.58 g of a styrene / carbon monoxide alternating copolymer product was obtained. The molecular weight of the obtained polymer product is M n = 47.2 kg / mol, the dispersion index is PDI = 1.10.
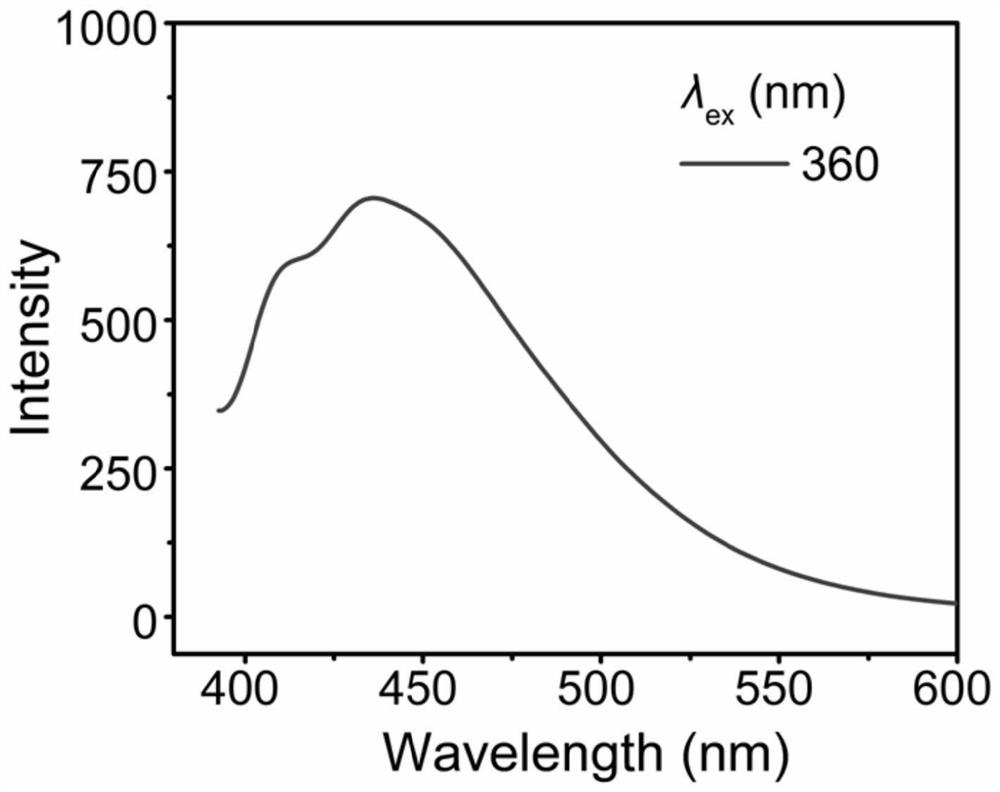
[0090] (2) In the same method as in Example 2, configure the tetrahydrofuran solution (0.1mol / L) of styrene / carbon monoxide alternating copolymers for fluorescence testing, which can emit blue fluorescence (maximum emission is 445nm) under 360nm excitation, see attached Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com