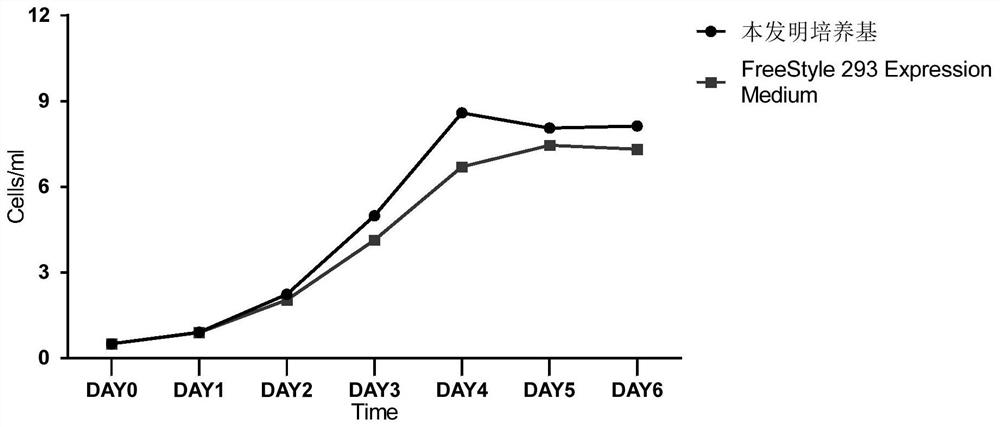
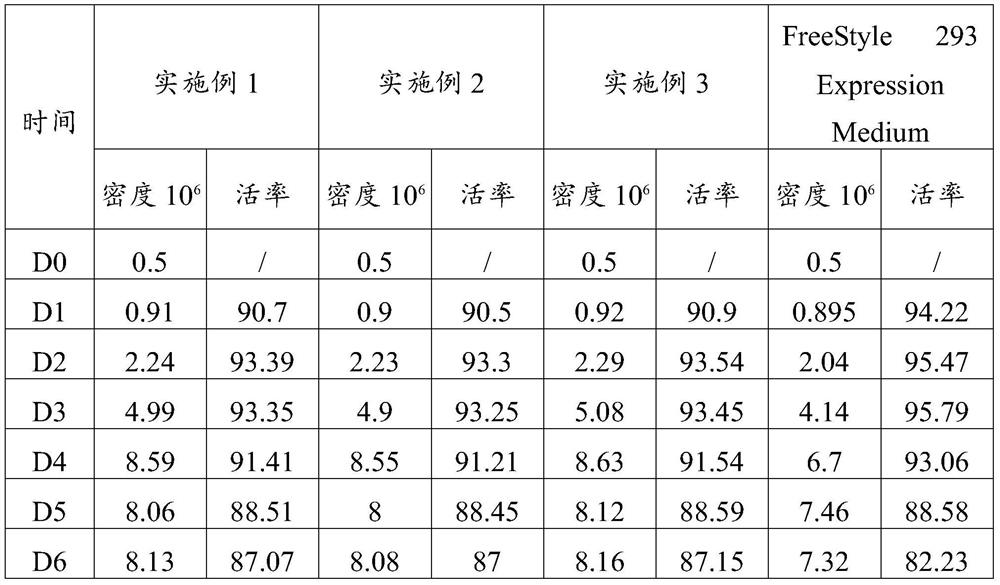
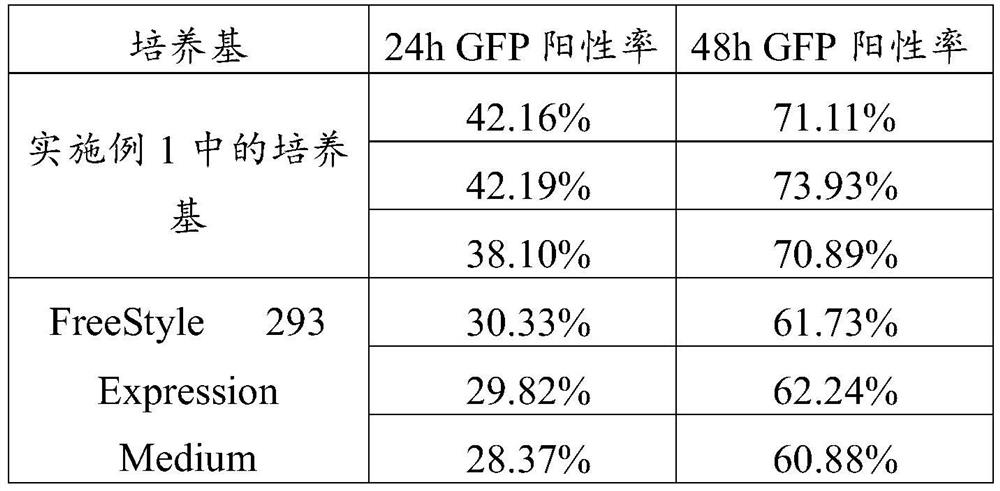
Serum-free culture medium for large-scale production of virus vectors as well as preparation method and use of serum-free culture medium
A serum-free medium and virus production technology, applied in biochemical equipment and methods, virus/bacteriophage, culture process, etc., can solve the problem of low virus recovery rate, achieve easy storage, reduce burden and cost, and ensure growth status and the effect of toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention also provides a method for preparing a serum-free medium for large-scale production of viral vectors as described above, which is characterized in that it comprises the following steps:
[0028] Weigh each component in proportion, dissolve in double distilled water, adjust the pH value, filter and sterilize to obtain the serum-free medium for large-scale production of virus vectors;
[0029] Save for later use.
[0030] Optionally, the range of the pH value is 7.0-7.4. For example but not limited to, the pH value of the serum-free medium for the large-scale production of viral vectors is 7.2.
[0031] Optionally, the specific condition of the preservation is: the serum-free culture for the large-scale production of the virus vector is preserved at 2-8°C. For example but not limited to, the serum-free culture for large-scale production of viral vectors is based on preservation at 4°C.
[0032] The present invention also provides an application of...
Embodiment 1
[0035] The preparation of the serum-free medium of embodiment 1 large-scale production virus vector:
[0036] S1. Weigh each component of the corresponding serum-free medium, each component is as follows: glycine 45mg / L, L-alanine 55mg / L, L-valine 95mg / L, L-leucine 230mg / L, L-isoleucine 125mg / L, L-methionine 55mg / L, L-proline 230mg / L, L-tryptophan 60mg / L, L-serine 410mg / L, L -Tyrosine 145mg / L, L-cysteine 25mg / L, L-phenylalanine 355mg / L, L-asparagine 35mg / L, L-aspartic acid 40mg / L, L-glutamine Aminoamide 1000mg / L, L-threonine 225mg / L, L-glutamic acid 80mg / L, L-lysine 245mg / L, L-arginine 75mg / L, L-histidine 60mg / L , vitamin D2 0.8mg / L, vitamin B2 0.08mg / L, vitamin B1 0.06mg / L, vitamin B6 0.4mg / L, vitamin A 0.6mg / L, vitamin E 0.035mg / L, vitamin B12 45mg / L, vitamin C12mg / L, choline chloride 3.5mg / L, folic acid 0.65mg / L, anhydrous magnesium sulfate 65mg / L, potassium chloride 500mg / L, sodium bicarbonate 1200mg / L, sodium chloride 8500mg / L, hydrogen phosphate Disodium 800mg / L, fe...
Embodiment 2
[0039] The preparation of the serum-free medium of embodiment 2 large-scale production virus vector:
[0040]S1. Weigh each component of the corresponding serum-free medium, each component is as follows: glycine 40mg / L, L-alanine 50mg / L, L-valine 90mg / L, L-leucine 200mg / L, L-isoleucine 100mg / L, L-methionine 50mg / L, L-proline 200mg / L, L-tryptophan 50mg / L, L-serine 400mg / L, L -Tyrosine 120mg / L, L-cysteine 20mg / L, L-phenylalanine 300mg / L, L-asparagine 30mg / L, L-aspartic acid 35mg / L, L-glutamine Aminoamide 800mg / L, L-threonine 200mg / L, L-glutamic acid 60mg / L, L-lysine 200mg / L, L-arginine 60mg / L, L-histidine 50mg / L , vitamin D2 0.6mg / L, vitamin B2 0.06mg / L, vitamin B1 0.04mg / L, vitamin B6 0.3mg / L, vitamin A0.5mg / L, vitamin E 0.030mg / L, vitamin B12 40mg / L, vitamin C10mg / L, choline chloride 3.0mg / L, folic acid 0.55mg / L, anhydrous magnesium sulfate 60mg / L, potassium chloride 480mg / L, sodium bicarbonate 1000mg / L, sodium chloride 8000mg / L, hydrogen phosphate Disodium 700mg / L, ferri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com