Lung-heat-clearing pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of excessive precipitation, large oral dosage, inaccurate dosage, etc., and achieve the effect of increasing the effect of nourishing lung yin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
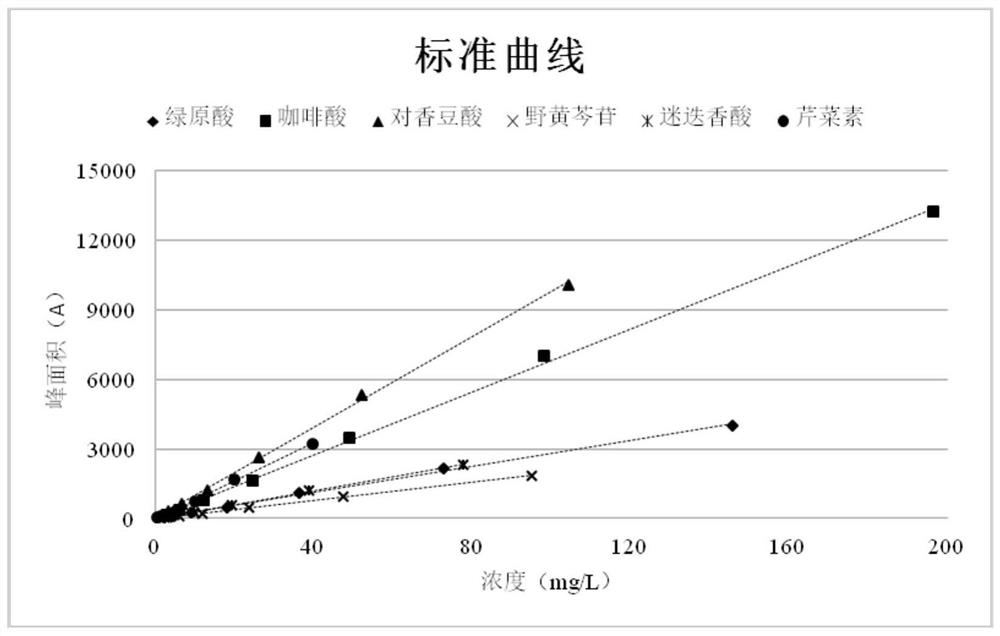
[0021] Example 1: Establish the fingerprints of chlorogenic acid, caffeic acid, p-coumaric acid, scutellarin, rosmarinic acid, and apigenin
[0022] When selecting the evaluation index of extraction rate, the active ingredient p-coumaric acid in Hedyotis diffusa, which is also the active ingredient in Scutellaria barbata; Fritillin B. Chlorogenic acid, caffeic acid, scutellarin, rosmarinic acid, and apigenin are active ingredients in other medicinal materials. Such selection can more comprehensively reflect the efficiency of Qingfei oral liquid reflux extraction.
[0023] 1.1 Preparation of reference substance stock solution
[0024] Precisely weigh 4.37 mg and 5.90 mg of the reference substances of chlorogenic acid and caffeic acid, respectively, and place them in 5ml measuring bottles; 5.71, 4.67, and 2.38mg were placed in 10ml measuring bottles, dissolved in methanol and diluted to the mark, and shaken to obtain concentrations of 0.874mg / ml, 1.18mg / ml, 0.626mg / ml, and 0....
Embodiment 2
[0044] Embodiment 2: peiminin A, peiminin B content assay method
[0045] Chromatographic conditions: chromatographic column is Kromasil C18, mobile phase: acetonitrile-water-diethylamine (70:30:0.03), flow rate: 0.7ml / min, column temperature: 30°C, evaporative photodetector, drift tube temperature: 45 °C, nitrogen flow rate 1.5L / min. External standard two-point determination method: Precisely draw 10 μl and 20 μl of the reference solution and 10 μl of the test solution respectively, inject them into the liquid chromatograph, and measure. Use the logarithmic equation to calculate the contents of peimin A and peimin B respectively. Preparation of the test solution: Rotate the test solution in 1.2 at 80°C to 90ml to obtain a concentrated medicinal solution containing 2.5g of crude drug per milliliter. Accurately draw 10ml of the concentrated medicinal solution, place it in a flask, and add 0.8 g of the concentrated ammonia test solution. ml soaked for 1.5 hours, precisely adde...
Embodiment 3
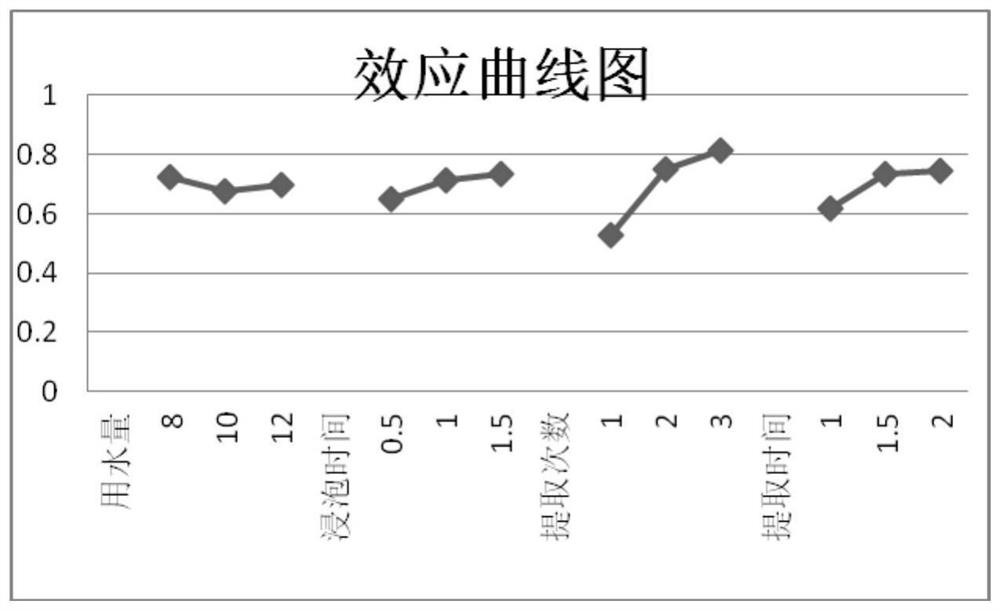
[0046] Embodiment 3: Orthogonal test investigation of extraction process conditions of Qingfei Oral Liquid
[0047] 3.1 Transfer rate
[0048] The transfer rate of 8 active ingredients including chlorogenic acid, caffeic acid, p-coumaric acid, scutellarin, rosmarinic acid, apigenin, peimin A and peimin B were used as evaluation indexes to investigate the preparation process.
[0049] 3.2 Anointing rate
[0050] The extraction rate of traditional Chinese medicine mixture refers to the percentage of the weight of the extract after concentrating the Chinese medicinal liquid into the extract to the weight of the original medicinal material. The extraction rate of the traditional Chinese medicine can indicate the extraction efficiency of the traditional Chinese medicinal material. Determination method: Take 200ml of the test solution in an evaporating dish dried to constant weight, evaporate to dryness in a water bath at 80°C, transfer the evaporating dish to a drying oven, dry at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com