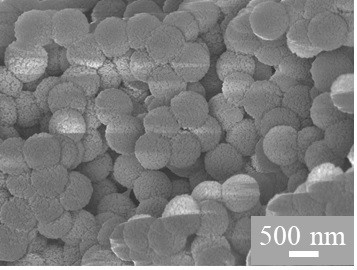
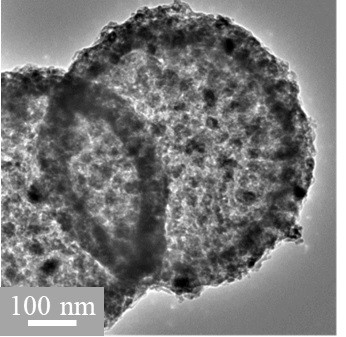
Hollow-structured transition metal cobalt and nitrogen co-doped carbon oxygen reduction catalyst as well as preparation method and application thereof
A transition metal and catalyst technology, applied in the field of catalyst preparation, can solve the problems of insufficient exposure of active sites, easy collapse of structure, and influence on catalytic activity, so as to promote exposure and mass transfer of reactants, low cost, and oxygen reduction catalysis Excellent active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Using sulfonated polystyrene microspheres with zeolite imidazolate framework compound material (ZIF-67) grown on the surface as a precursor, cobalt and nitrogen co-doped carbon oxygen reduction catalysts are prepared by high-temperature pyrolysis carbonization, including the following steps:
[0035]Styrene monomer and divinylbenzene monomer were washed with sodium hydroxide solution with a mass fraction of 10%, and then styrene was washed to neutrality with deionized water; 0.7 g of crosslinking agent divinylbenzene was dissolved in 120 Add 14 g of styrene in mL of ultrapure water, stir evenly, heat and react for 1 h under an inert atmosphere, dissolve 0.03 g of potassium persulfate in 10 mL of deionized water, add it to the reaction system, and continue the reaction for 10 h; Saturated NaCl solution was added to the reaction product, and the precipitate was filtered and dried to obtain polystyrene microspheres. Mix 1.70 g polystyrene microsphere template and 60 mL con...
Embodiment 2
[0039] The operating conditions were the same as in Example 1, except that the reaction time was increased to 18 h during the preparation of the precursor. Oxygen reduction performance test of catalyst prepared by pyrolytic carbonization ( Figure 4 ) indicates that the catalyst still exhibits excellent catalytic activity for oxygen reduction.
Embodiment 3
[0041] The operating conditions were the same as in Example 1, except that the reaction time was increased to 22 h during the preparation of the precursor. Oxygen reduction performance test of catalyst prepared by pyrolytic carbonization ( Figure 4 ) indicates that the catalyst still exhibits excellent catalytic activity for oxygen reduction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com